Enteric sustained-release preparation with aspirin and bisoprolol as active ingredients
A technology of aspirin and bisoprolol, applied in the field of medicine, can solve the problems of increased gastric damage and large losses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
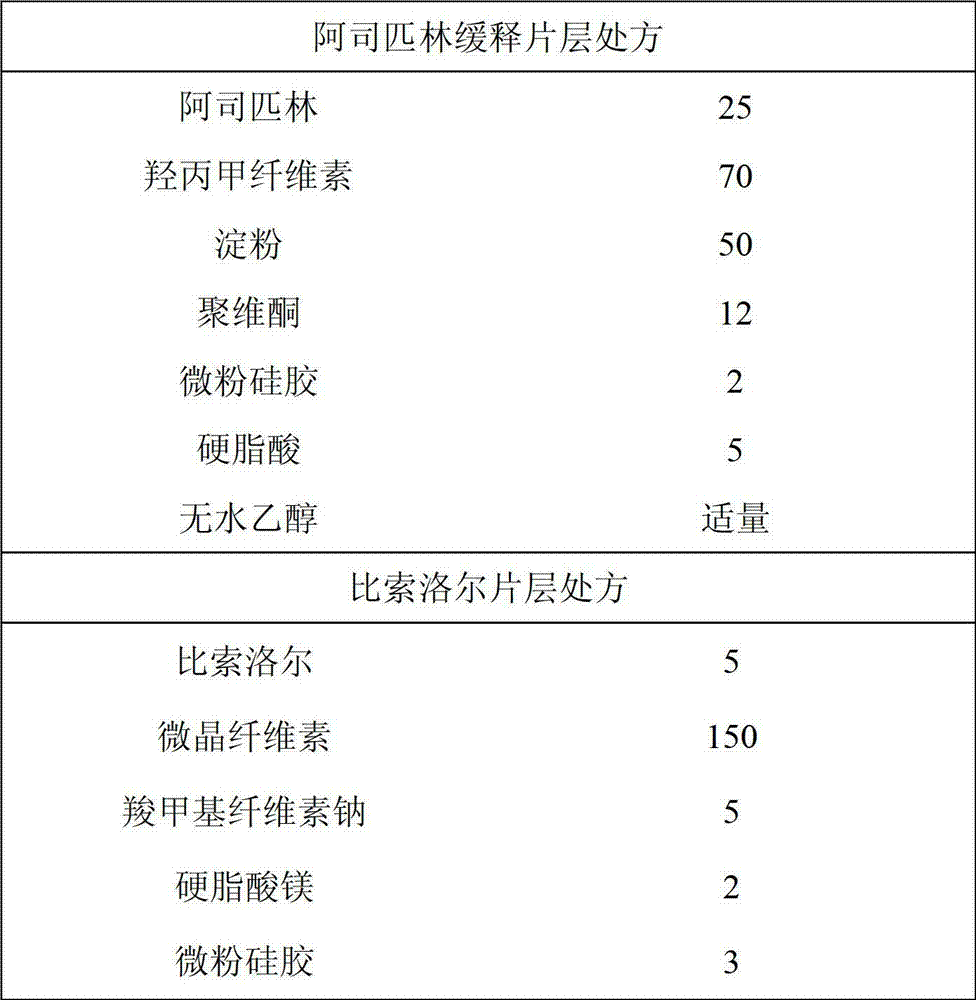
[0014] Aspirin Extended Release and Bisoprolol Bilayer Tablets
[0015]
[0016] Preparation method: aspirin crushed, spare. Povidone was dissolved in absolute ethanol for later use. Mix aspirin and auxiliary materials evenly, add binder (povidone absolute ethanol solution) to granulate, dry, and granulate. The bisoprolol is pulverized, and other auxiliary materials are added and mixed evenly. The two active ingredient material mixtures are respectively placed in two different feeding hoppers, and a double-layer tablet pressing process is adopted to prepare the compound double-layer tablet of the target preparation.
Embodiment 2
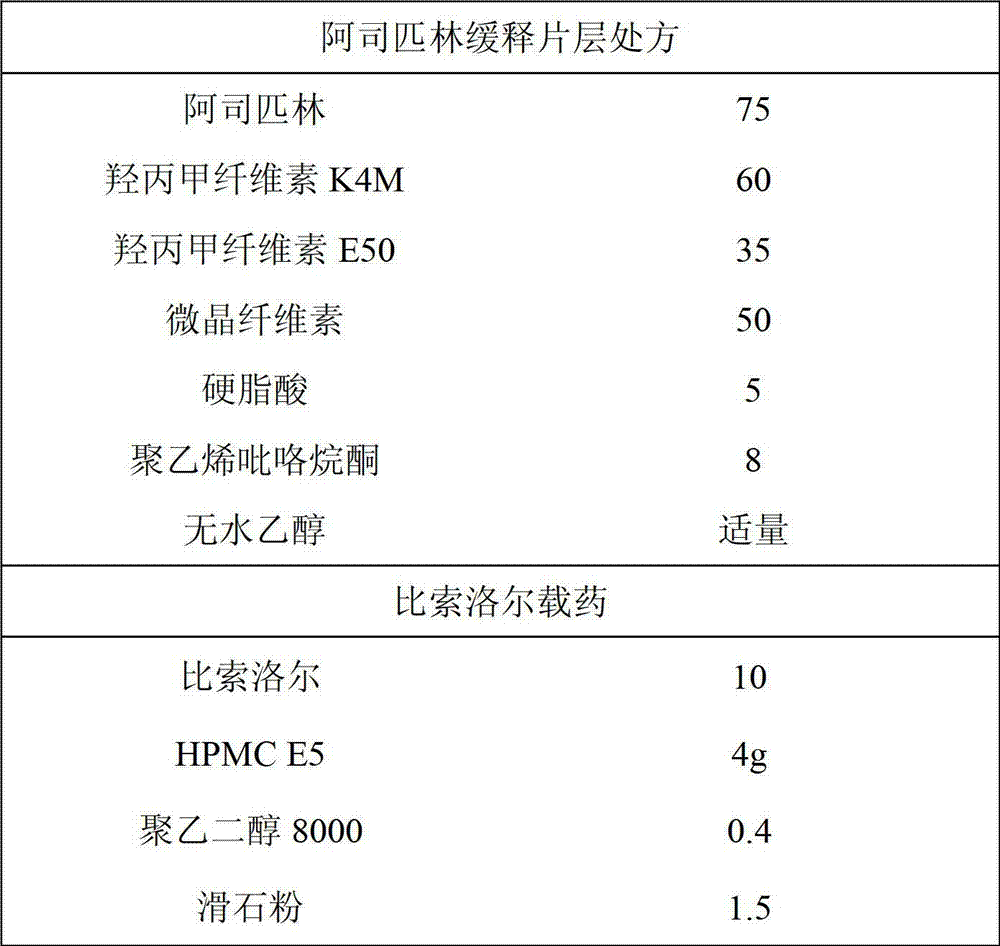
[0018] Aspirin Extended Release and Bisoprolol Film Coated Tablets
[0019]
[0020] Preparation method:
[0021] 1. Crush the aspirin and keep it for later use. Polyvinylpyrrolidone was dissolved in ethanol for later use. Mix the aspirin and auxiliary materials evenly, add polyvinylpyrrolidone absolute ethanol solution to granulate, dry, granulate, and compress into tablets.
[0022] 2. Dissolve HPMC E5 and polyethylene glycol 8000 in water, add bisoprolol and talcum powder, and homogenize through a colloid mill for about 5 minutes.
[0023] 3. Put the aspirin tablets into the coating pan, adjust the air inlet temperature to keep the material temperature at about 45°C, then adjust the spray gun atomization pressure, air flow rate and liquid medicine flow rate, and spray it to get the product
Embodiment 3
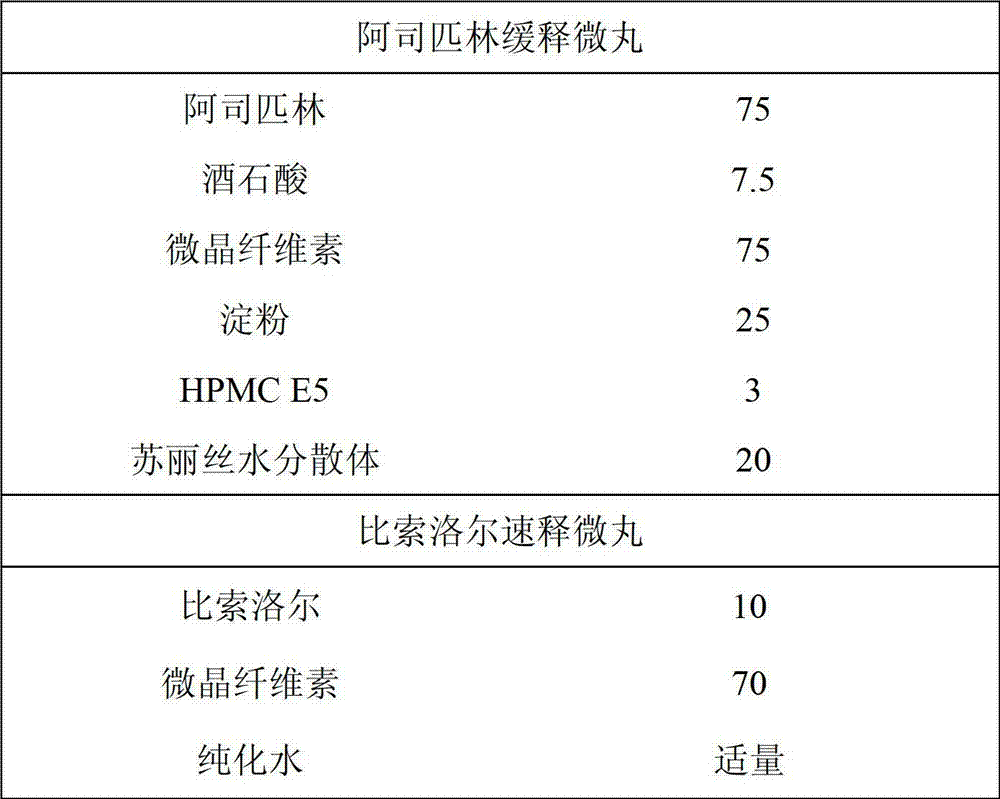
[0025] Aspirin enteric-coated sustained-release pellets and bisoprolol immediate-release pellets
[0026]
[0027] Preparation method:
[0028] 1. Grind aspirin and tartaric acid, add microcrystalline cellulose, starch and other auxiliary materials and mix well; set aside
[0029] 2. Configure 20% starch slurry, and use starch slurry as a binder to prepare soft materials.
[0030] 3. Put the soft material into the extruding spheronizer, extrude it into a long strip, and then put it into the shot blasting machine, adjust the rotation at 600-800rpm, and perform shot blasting to get small pellets with a particle size between 40-12 mesh , sieve, and take the drug-loaded pills with a particle size between 24-12 mesh
[0031] 4. Put the above drug-loaded pellets into the fluidized bed (Shenzhen Xinyite, MINI fluidized bed), adjust the material temperature to about 40°C, the inlet air temperature to 80-100°C, the atomization pressure and fluidization pressure 0.1Mpa respectivel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com