Rh3Ni1 catalyst and method for preparing arylamine by carrying out catalytic reduction on nitro aromatic hydrocarbon through Rh3Ni1 catalyst
A technology of nitroaromatics and catalysts, applied in the field of heterogeneous catalysis, can solve problems such as complex structures, and achieve the effects of simple preparation procedures, high catalyst activity and selectivity, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
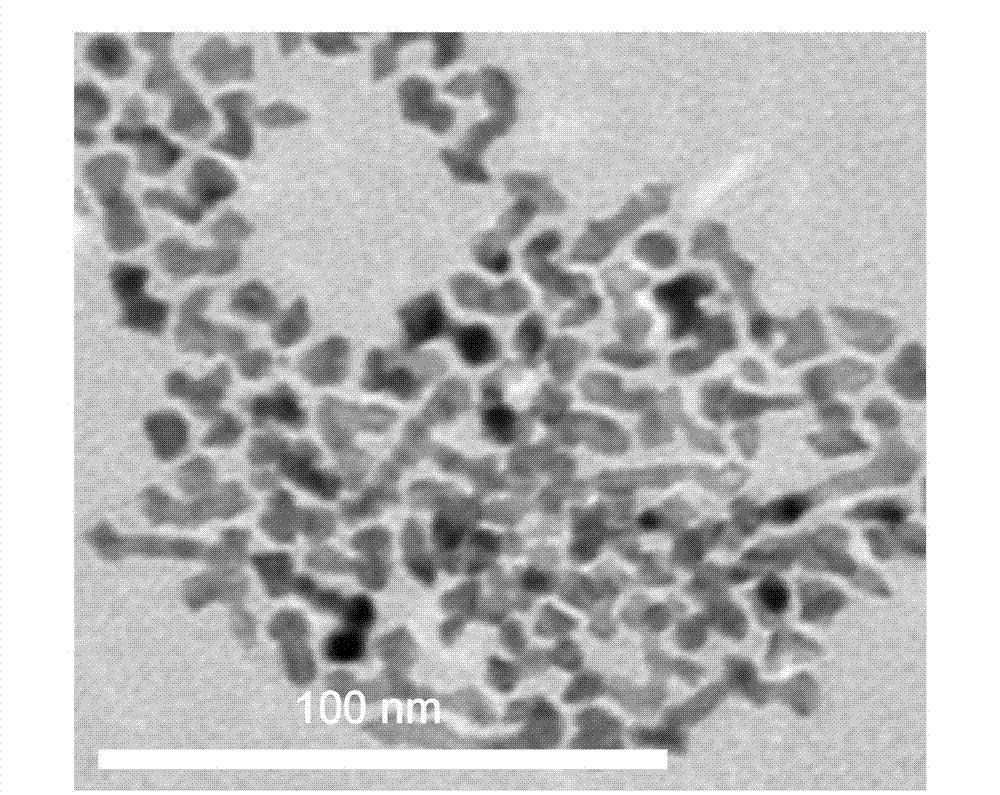
[0041]Implementation method: heat octadecylamine to form a clear solution as solvent, surfactant and reducing agent. Continue heating to 140 DEG C, then add trihydrate rhodium trichloride and nickel acetylacetonate, wherein the mass molar concentration of trihydrate rhodium trichloride is 0.045mmol / g, and the mass molar concentration of nickel acetylacetonate is 0.015mmol / g, A clear liquid was obtained. In the state of vigorous stirring, inject the above-mentioned clear liquid into octadecylamine that has been warmed up to 240°C in advance for dilution, and the mass molar concentration of rhodium trichloride trihydrate is 0.012mmol / g, and the mass molar concentration of nickel acetylacetonate is 0.012mmol / g. A solution with a molar concentration of 0.004mmol / g. Aging the above solution for 3 minutes at 240°C, cooling to 70°C, adding ethanol to precipitate a precipitate, washing with ethanol after centrifugation to obtain bimetallic Rh 3 Ni 1 , wherein the particle size of t...
Embodiment 3
[0043] Implementation method: heat octadecylamine to form a clear solution as solvent, surfactant and reducing agent. Continue heating to 150 DEG C, then add trihydrate rhodium trichloride and nickel acetylacetonate, wherein the mass molar concentration of trihydrate rhodium trichloride is 0.075mmol / g, and the mass molar concentration of nickel acetylacetonate is 0.025mmol / g, A clear liquid was obtained. In the state of vigorous stirring, inject the above-mentioned clear liquid into octadecylamine that has been warmed up to 250°C in advance for dilution, and the mass molar concentration of rhodium trichloride trihydrate is 0.015mmol / g, and the mass molar concentration of nickel acetylacetonate is 0.015mmol / g. A solution with a molar concentration of 0.005mmol / g. Aging the above solution at 250°C for 5 minutes, cooling to 80°C, adding ethanol, precipitated precipitate, washing with ethanol after centrifugation to obtain bimetallic Rh 3 Ni 1 , where the particle size of the s...
Embodiment 4
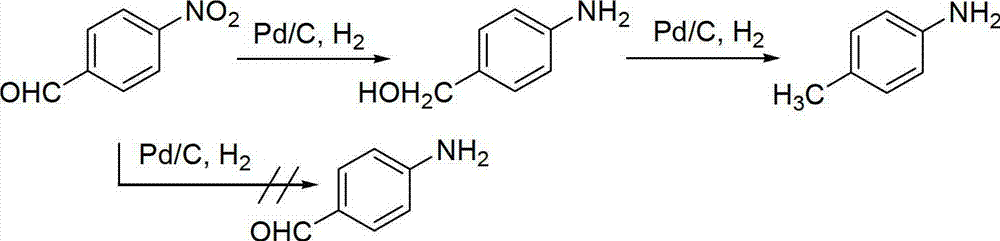
[0045] Implementation method: at room temperature, mix nitrobenzene and Rh 3 Ni 1 Place in ethyl acetate, wherein the molar concentration of the substrate is 0.05mol / L, and the molar concentration of the catalyst is 0.00015mol / L. After sealing the reactor, add a balloon filled with hydrogen, and continuously change the air for 3 times. Under the state of stirring, react the mixture at room temperature for 8 hours, recover the catalyst by centrifugation, and distill the supernatant to remove the solvent under reduced pressure to obtain aniline. The yield 99%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystal size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com