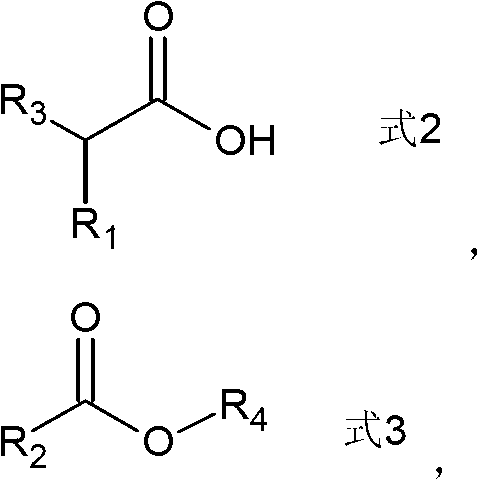
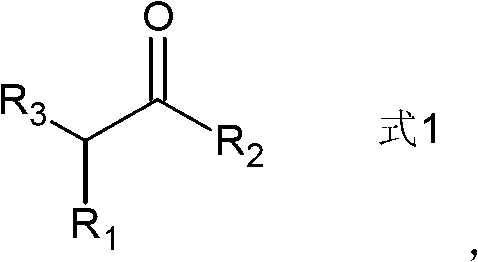
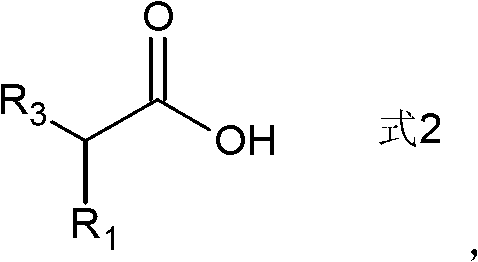
Preparation method of ketone compound and its derivatives
A technology for ketone compounds and carboxylic acid compounds, applied in the field of preparation of ketone compounds and derivatives thereof, can solve the problems of complex operation, poor selectivity, long synthesis steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0159] The preparation and characterization of embodiment 1. diphenyl ethyl ketone
[0160]
[0161] Add 0.136g (1.0mmol) of phenylacetic acid (1.0mmol) and 0.136g (1.0mmol) of methyl benzoate to a dry 25mL schlenk bottle, 4mL of anhydrous DMF, and slowly add 2.0mL (4.0mmol, 4.0eq) at -10°C Sodium hexamethyldisilazide (NaHMDS) (5min) (4.0mmol), keep warm and continue stirring for 3.5h, monitor the reaction by TLC, after the reaction is completed, add saturated ammonium chloride (10mL) to quench the reaction, extract with ethyl acetate Three times, anhydrous Na 2 SO 4 Drying, rotary evaporation, and separation by column chromatography gave the target product as white crystals with a yield of 90%. mp=127-128℃. 1 H-NMR (300MHz, CDCl 3 ):δ8.05(s,1H),8.02(t,J=1.5Hz,1H),7.60-7.54(m,1H),7.47(t,J=7.2Hz,2H),7.37-7.24(m, 5H),4.31(s,2H); 13 C-NMR (75MHz, CDCl 3 ):δ197.7,136.6,134.6,133.2,130.2,129.5,128.7,128.7,128.6,128.5,126.9,45.5.IR(KBr,cm -1 ):3042,2904,1686,1596,1449,133...
Embodiment 2
[0162] Example 2. Preparation and characterization of 3-methoxyphenyl-2-(2-methoxyphenyl)ethanone
[0163]
[0164] Using 2-methoxyphenylacetic acid (1.0 mmol), methyl 3-methoxybenzoate (1.0 mmol), NaHMDS (4.0 mmol), saturated ammonium chloride (10 mL), by a similar method as described in Example 1 The target compound was prepared as a colorless oily liquid with a yield of 86%. 1 H-NMR (500MHz, CDCl 3 ):δ7.65(d,J=7.5Hz,1H),7.57(s,1H),7.36(t,J=7.5Hz,1H),7.26(d,J=8.0Hz,1H),7.18(d ,J=7.5Hz,1H),7.10(dd,J=8.0Hz,J=2.5Hz,1H),6.94(d,J=8.0Hz,1H),6.90(t,J=8.0Hz,1H), 4.27(s,2H),3.84(s,3H),3.79(s,3H); 13 C-NMR (75MHz, CDCl 3 ):δ198.0,157.3,137.1,132.9,131.0,129.6,128.5,128.4,128.3,123.8,120.7,110.6,55.4,40.0.IR(KBr,cm -1 ):3068,2975,2948,2841,1691,1601,1498,1194,1013.HRMS(ESI)C 16 h 16 o 3 ([M+H] + )257.1099, found 257.1173.
Embodiment 3
[0165] Example 3. Preparation and characterization of 2-(3,4-dichlorophenyl)-1-(2-furyl)-ethanone
[0166]
[0167] Using 3,4-dichlorophenylacetic acid (1.0mmol), methyl 2-furancarboxylate (1.0mmol), NaHMDS (4.0mmol), saturated ammonium chloride (10mL), the target was prepared by a method similar to that described in Example 1 Compound, light yellow oily liquid, yield 71%. 1 H-NMR (500MHz, CDCl 3 ):δ7.61(s,1H),7.40(d,J=1.5Hz,1H),7.38(d,J=8.0Hz,1H),7.25(t,J=6.0Hz,1H),7.13(dd ,J=11.5Hz,J=2.0Hz,1H),4.08(s,2H); 13 C-NMR (75MHz, CDCl 3 ):δ185.4,152.2,146.8,134.1,132.5,131.5,131.3,130.5,129.0,118.0,112.6,44.1.IR(KBr,cm -1 ):3135,2931,1672,1568,1466,1133,1033.HRMS(ESI)calcd.for C 12 h 8 C 12 o 2 ([M+H] + )254.9901, found 254.9977.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com