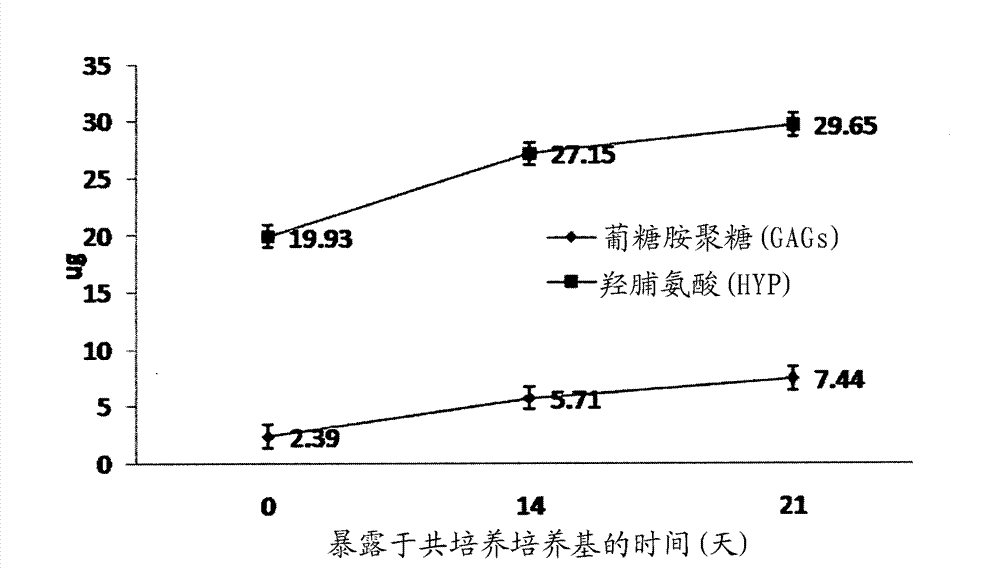
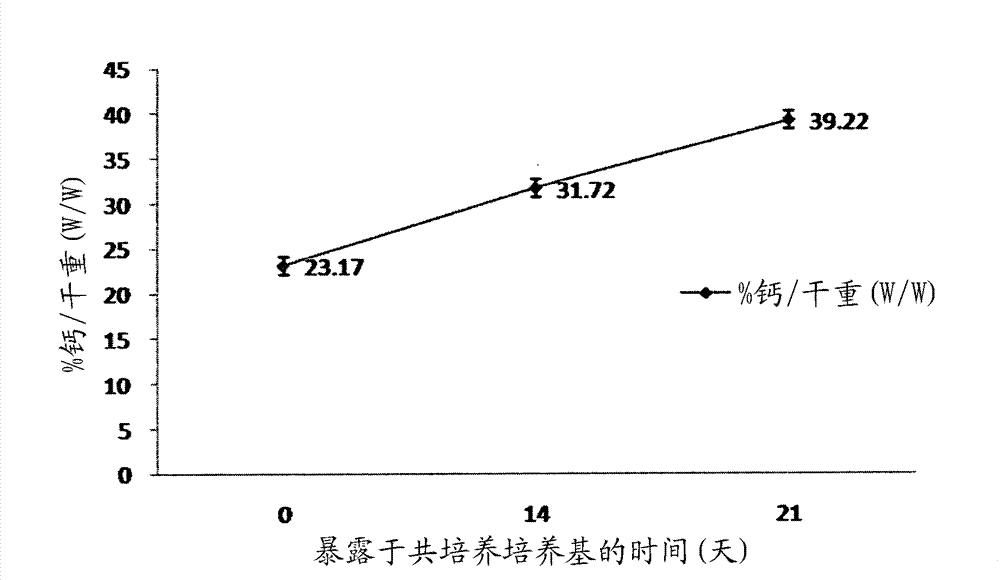
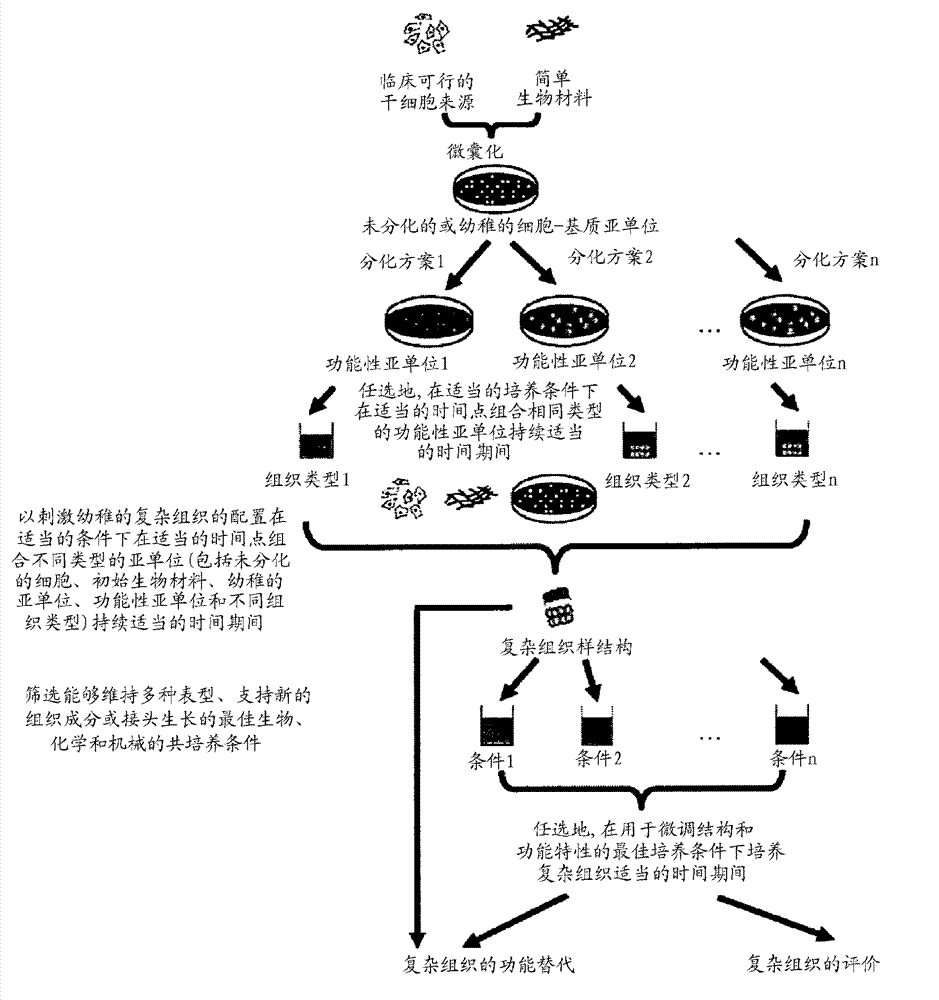
Methods for complex tissue engineering
A complex tissue and bioengineering technology, applied in biochemical equipment and methods, tissue culture, bone/connective tissue cells, etc., can solve problems such as inability to prepare
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1: Bone marrow aspiration and separation of rabbit bone marrow mesenchymal stem cells (rMSC)
[0075] Three-month-old New Zealand White rabbits weighing an average of 3.5 kg were anesthetized by intramuscular injection of a mixture of 10% ketamine hydrochloride (0.35 ml / kg) and 2% xylazine (0.25 ml / kg). About 5 ml of bone marrow was aspirated from the tibia. After Ficoll-Hypague gradient separation, monocytes at the interface were collected and cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) and antibiotics. The medium was refreshed 10 days after inoculation and refilled every 2 days thereafter. Visible colonies of adherent cells were found approximately 5-7 days after initial plating. After reaching confluence (approximately 12-14 days after initial plating), cells were detached by 0.25% trypsin / EDTA for subculture.
[0076] Alternatively, rMSCs can be purchased from Beijing YiKeLiHao Biotechnology Co., Ltd.
Embodiment 2
[0077] Embodiment 2: the culture of rMSC
[0078] In Dulbecco's modified Eagle medium (DMEM-HG) with high glucose, 10% fetal bovine serum (FBS), 100U / ml penicillin, 100mg / ml streptomycin, 1.875mg / ml sodium bicarbonate (NaHCO 3 ), 0.02M HEPES and 0.29mg / ml L-glutamine to culture rMSC in the complete medium. The final pH of the medium was adjusted to 7.4 with 1 N sodium hydroxide (NaOH). Live cells in culture were separated from dead cells after 24 hours by adhesion selection, ie, cells were cultured for 24 hours and adherent cells were separated from dead cells in culture. Maintain cells in complete medium and refill every 3 days. Subconfluence rMSCs were detached by 0.25% trypsin / EDTA. Cells from passage 2-3 were used in the subsequent microencapsulation step.
Embodiment 3
[0079] Example 3: Fabrication of Naïve Subunit-Collagen-rMSC Microspheres
[0080] Ice-cold rat tail type I collagen (Becton Dickenson) was neutralized with 1 N NaOH and further diluted with complete medium to a final concentration of 2 mg / ml. Aliquots of rMSCs from P2-P3 in complete medium were quickly mixed with neutralized collagen solution in an ice bath, resulting in a cell-matrix mixture with a final cell density of 1250 cells per 2.5 μl drop. Droplets were dispensed into 35mm diameter Petri dishes (Sterlin) with UV irradiated parafilm on the bottom layer. With 5% CO 2 After incubation for 1 hour at 37°C in a humidified atmosphere, the droplets gelled to form solid rMSC-collagen microspheres, which were then gently rinsed into Petri dishes using complete medium and incubated for 3 hours prior to the differentiation step. sky.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com