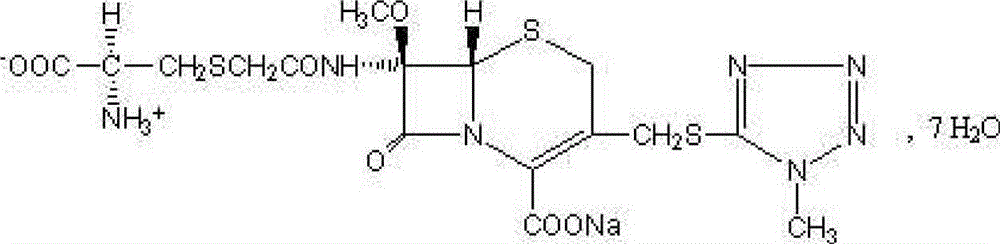
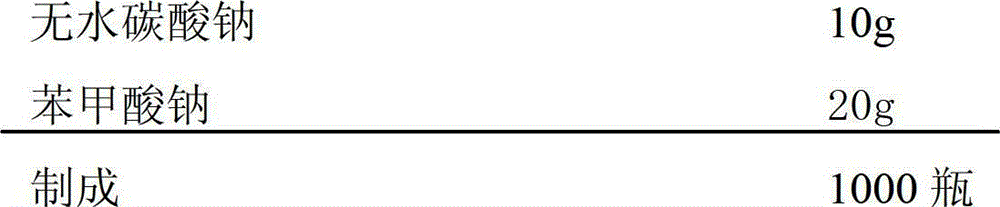Pharmaceutical composition with cefminox sodium sterile mixed powder form
A technology of cefminox sodium and its composition, which is applied in the field of pharmaceutical compositions in the form of cefminox sodium aseptic mixed powder, can solve the problems of increased risk of allergic reactions, increased polymer content, etc. Safety and effectiveness, improved stability, good thermal stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
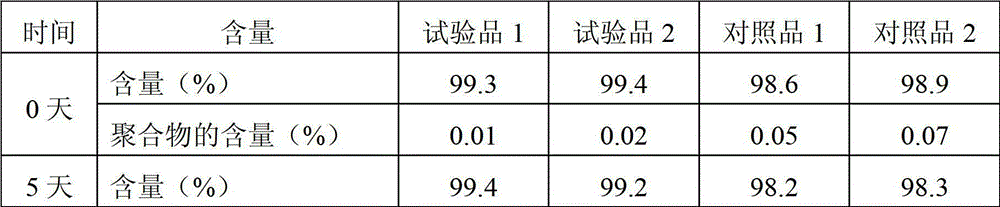
Examples
Embodiment 1
[0040] [Example 1] Cefminox Sodium Sterile Powder for Injection
[0041] Specification:
[0042] Preparation:
[0043] 1. Inner packaging material treatment
[0044] Antibiotic glass bottles, rubber stoppers, and aluminum caps are cleaned, dried, and sterilized according to the conventional process, and set aside;
[0045] 2. Specific steps
[0046] (1) Weigh the prescribed amount of anhydrous sodium carbonate, anhydrous sodium carbonate, and sodium benzoate, and mix them evenly in a sterile container;
[0047] (2) Inspection of intermediate products;
[0048] (3) Aseptic subpackaging according to specifications;
[0049] (4) Plugging and capping;
[0050] (5) Packaging, full inspection, and storage.
Embodiment 2
[0051] [Example 2] Cefminox Sodium Sterile Powder Injection
[0052] Specification:
[0053] Preparation method: same as preparation example 1.
Embodiment 3
[0054] [Example 3] Cefminox Sodium Sterile Powder Injection
[0055] Specification:
[0056] Preparation method: with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com