Preparation for glipizide compression-coated controlled-release tablets
A technology for glipizide and coated tablets, which is applied in the field of glipizide compression-coated controlled-release tablets, can solve the problems of unfavorable large-scale production, cumbersome process and high cost, and achieves improvement of peristaltic promotion effect and maintenance of blood medicine. effect of concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
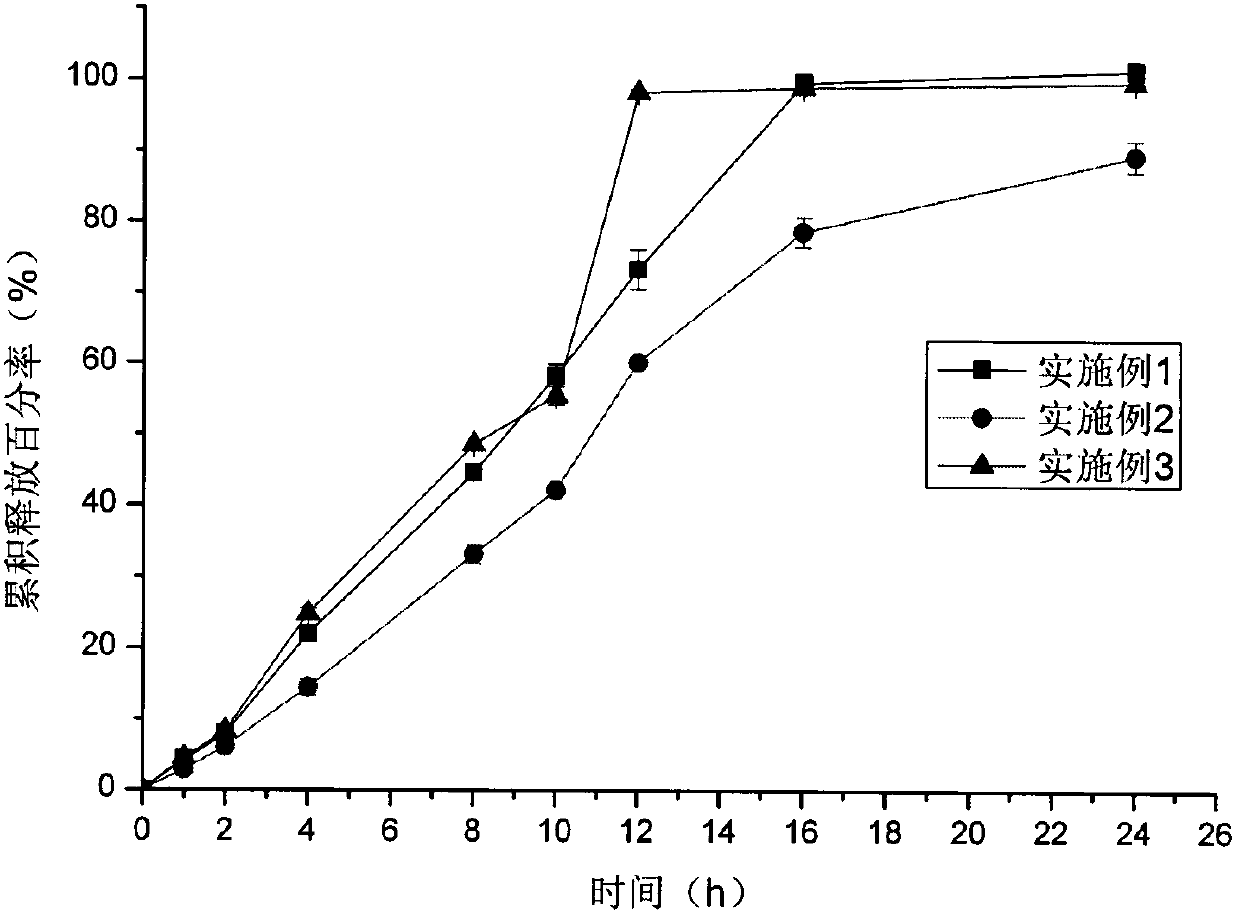
Embodiment 1
[0028] Chip
[0029]
[0030]The above-mentioned raw materials (excluding magnesium stearate) are uniformly mixed, dried at 60°C after granulation, sieved and granulated, and then magnesium stearate is added and mixed evenly. Tablets were compressed with a single-punch tablet machine under a pressure of 30N to form core tablets (diameter=7mm) each weighing 94.5 mg. Shell
[0031]
[0032] The above-mentioned raw materials (excluding magnesium stearate) are uniformly mixed, dried at 60°C after granulation, sieved and granulated, and then magnesium stearate is added and mixed evenly. Dry-coating machine (Kikusui Cleanpress Corret 18DC) tableting under 90N pressure, the shell layer and pre-prepared tablet core were combined to form compression-coated tablets (diameter=9mm) containing glipizide 5mg each weighing 400mg .
Embodiment 2
[0034] Chip
[0035]
[0036] The above-mentioned raw materials (excluding magnesium stearate) are uniformly mixed, dried at 60°C after granulation, sieved and granulated, and then magnesium stearate is added and mixed evenly. Tablets were compressed with a single-punch tablet machine under a pressure of 30N to form core tablets (diameter=7mm) each weighing 112 mg.
[0037] Shell
[0038]
[0039] The above-mentioned raw materials (excluding magnesium stearate) are uniformly mixed, dried at 60°C after granulation, sieved and granulated, and then magnesium stearate is added and mixed evenly. Dry-coating machine (Kikusui Cleanpress Corret 18DC) tableting under 90N pressure, the shell layer and pre-prepared tablet core were combined to form a press-coated tablet (diameter=9mm) containing glipizide 5mg each weighing 410mg .
Embodiment 3
[0041] Chip
[0042]
[0043] The above-mentioned raw materials (excluding magnesium stearate) are uniformly mixed, dried at 60°C after granulation, sieved and granulated, and then magnesium stearate is added and mixed evenly. Tablets were compressed with a single-punch tablet machine under a pressure of 30N to form core tablets (diameter=7mm) each weighing 187 mg.
[0044] Shell
[0045]
[0046] The above-mentioned raw materials (excluding magnesium stearate) are uniformly mixed, dried at 60°C after granulation, sieved and granulated, and then magnesium stearate is added and mixed evenly. Dry-coating machine (Kikusui Cleanpress Corret 18DC) tableting under 90N pressure, the shell layer and pre-prepared tablet core were combined to form compression-coated tablets (diameter=9mm) containing glipizide 5mg each weighing 480mg .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com