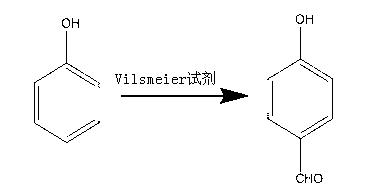
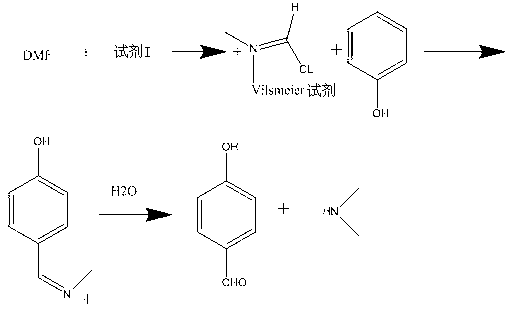
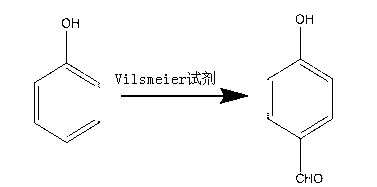
Synthesis method of p-hydroxybenzaldehyde
A technology of p-hydroxybenzaldehyde and its synthesis method, which is applied in the preparation of carbon-based compounds, chemical instruments and methods, and the preparation of organic compounds, and can solve the problems of high cost, difficulty in separation and purification, and low yield of p-hydroxybenzaldehyde , to achieve the effect of low equipment requirements, high yield and purity, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The synthetic method of p-Hydroxybenzaldehyde takes phenol as raw material, and concrete steps comprise:
[0028] (1) Add 300mL toluene to a dry four-neck flask, then add 100mL DMF, measure 93.79mL SOCl 2 In the constant pressure dropping funnel, add SOCl dropwise to the four-neck flask 2 , the reaction temperature is 30°C, and stirred for 30 minutes to obtain the Vilsmeier reagent;
[0029] (2) Weigh 121.68g of phenol into a four-necked flask, keep it warm at 60°C for 80min, then cool down to 50°C, add 250mL of water at 83°C and stir for 100min;
[0030] (3) Transfer the solution in the four-neck flask in step (2) into a separatory funnel and let it stand, cut off the lower aqueous phase, add water with a volume of 300mL and a temperature of 80°C for washing; then cut off the lower aqueous phase, and take Remove the toluene from the toluene phase under negative pressure and dry to obtain a light yellow solid, that is, 143.85 g of p-hydroxybenzaldehyde with a purity o...
Embodiment 2
[0032] The synthetic method of p-Hydroxybenzaldehyde takes phenol as raw material, and concrete steps comprise:
[0033] (1) Add 100mL toluene to a dry four-neck flask, then add 12.05mL POCL 3 , Measure 10mL of DMF in a constant pressure dropping funnel, add DMF dropwise to a four-necked flask at a reaction temperature of 10°C, stir for 45min, and obtain Vilsmeier reagent;
[0034] (2) Weigh 10.15g of phenol into a four-necked flask, keep it warm at 110°C for 30min, then cool down to 25°C, add 200mL of water at 0°C and stir for 100min;
[0035] (3) Transfer the solution in the four-neck flask in step (2) into a separatory funnel and let it stand, cut off the lower aqueous phase, add water with a volume of 280mL and a temperature of 60°C for washing; then cut off the lower aqueous phase, and take Remove the toluene from the toluene phase under negative pressure and dry to obtain a light yellow solid, that is, 12.21 g of p-hydroxybenzaldehyde with a purity of 98.7% and a yield ...
Embodiment 3
[0037] The synthetic method of p-Hydroxybenzaldehyde takes phenol as raw material, and concrete steps comprise:
[0038] (1) Add 200mL toluene to a dry four-neck flask, then add 48.61mL PCL 5 , Measure 50mL of DMF in a constant pressure dropping funnel, add DMF dropwise to a four-necked flask at a reaction temperature of 20°C, stir for 70min, and obtain Vilsmeier reagent;
[0039] (2) Weigh 60.84g of phenol, dissolve it in 100mL of toluene solution and add it to a four-necked flask, keep it at 85°C for 180min, then cool down to 40°C, add 150mL of water at 40°C and stir for 65min;
[0040] (3) Transfer the solution in the four-neck flask in step (2) into a separatory funnel and let it stand, cut off the lower aqueous phase, add water with a volume of 180mL and a temperature of 40°C for washing; then cut off the lower aqueous phase, and take Remove the toluene from the toluene phase under negative pressure and dry to obtain a light yellow solid, that is, 73.18 g of p-hydroxyben...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com