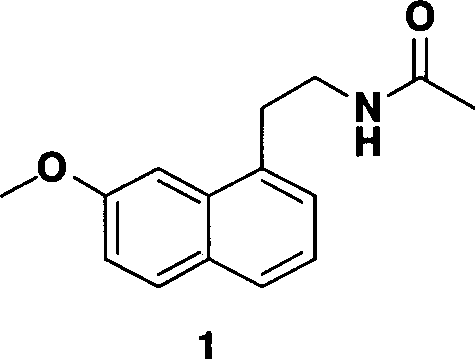
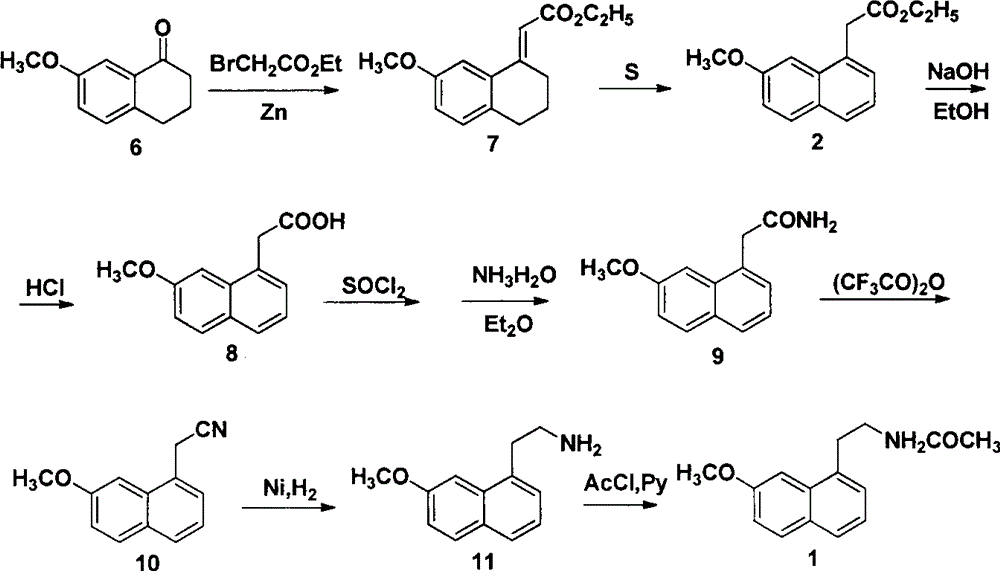
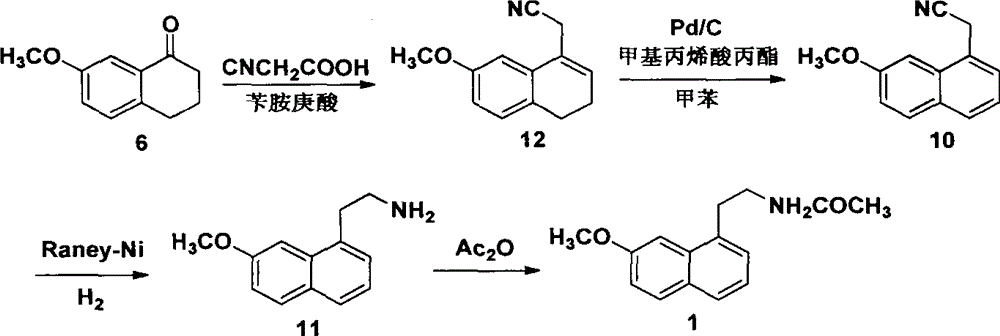
A new method for synthesizing agomelatine
A technology of agomelatine and compounds, applied in the field of pharmaceutical chemical synthesis, can solve the problems of unfavorable large-scale production and high requirements for production equipment, and achieve the effects of simple operation, high industrial application value, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1 prepares agomelatine
[0028] The preparation method of the present embodiment comprises the following steps:
[0029] The preparation of step 1 7-methoxy-1-naphthyl alcohol
[0030] 4.9 g of ethyl 7-methoxy-1-naphthalene acetate, NaBH 4 1.6g, ZnCl 2 2.7g of THF50ml solution was refluxed for 3 hours, TLC showed that the reaction was complete, cooled to room temperature, carefully added 100ml of dilute hydrochloric acid to quench the reaction; extracted with 200ml of ethyl acetate, combined organic layers, washed with saturated sodium chloride, dried over anhydrous sodium sulfate ; The solvent was distilled off under reduced pressure to obtain 3.8 g of an off-white solid with a melting point of 83-85° C. and a yield of 95%.
[0031] Preparation of step 2 7-methoxy-1-naphthyl ethyl methanesulfonate
[0032] 1.0 ml of methanesulfonyl chloride was added dropwise to a solution of 1.6 g of 7-methoxy-1-naphthyl ethanol and 1.5 ml of triethylamine in 25 ml of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com