Synthesizing method of tetrahydroquinoline derivative
A technology of tetrahydroquinoline and synthetic method, which is applied in the field of synthesis of tetrahydroquinoline derivatives, can solve the problems of highly corrosive production equipment, unfavorable mass production and application, and large consumption of accelerators, etc., and achieves low corrosiveness, The effect of low price and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
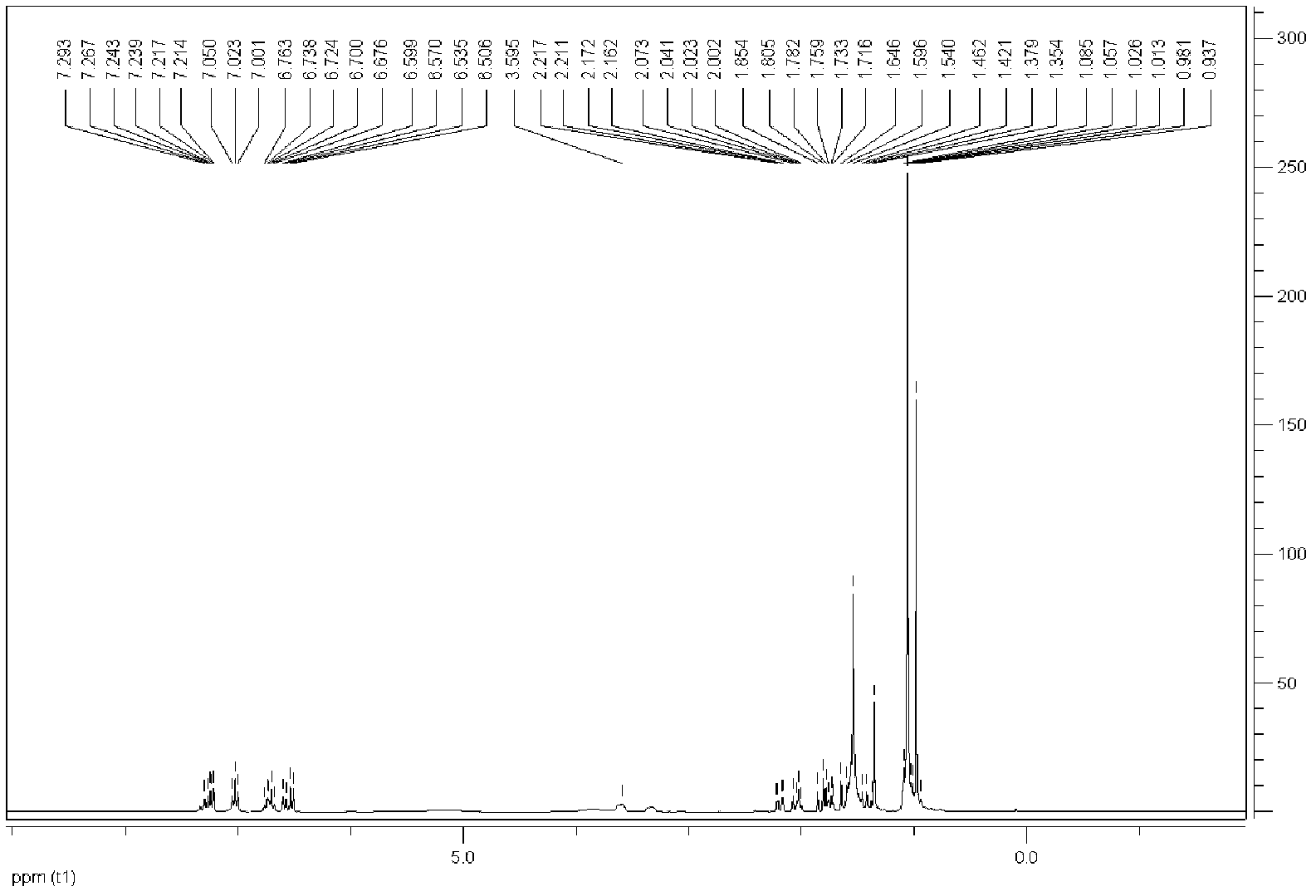
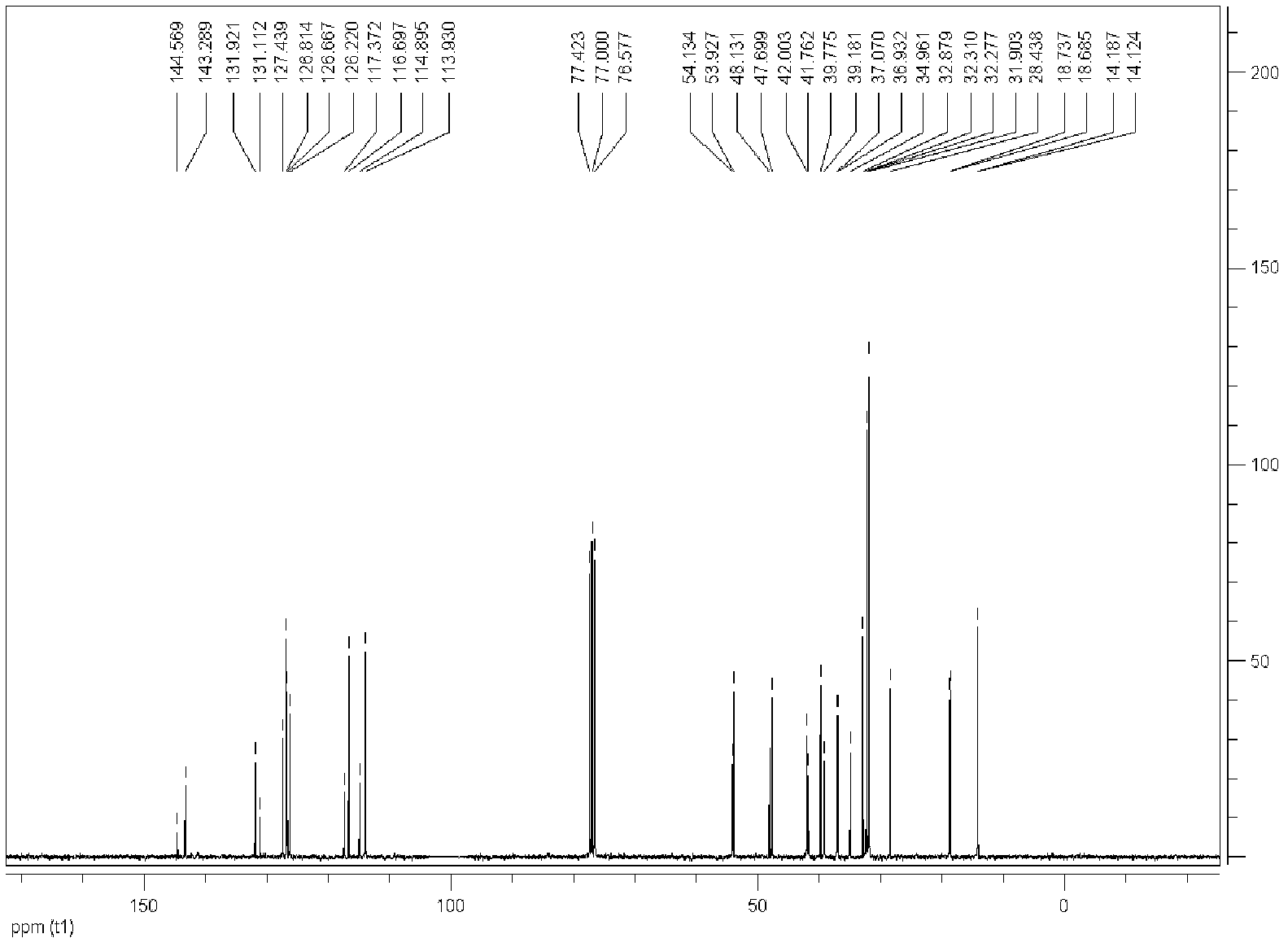
[0060] Embodiment 1: prepare 4-methyl-2-propyl group-4-neopentyl-1,2,3,4-tetrahydroquinoline (cis-D 1 +trans-D 1 )
[0061] SnCl 2 0.3792g (2mmol) and FeCl 3 Dissolve 0.6488g (4mmol) in 13mL of dichloromethane, stir at 40°C for 4 hours (h), add n-butyraldehyde 0.150mL (1.667mmol), aniline 0.152mL (1.667mmol), diisobutylene (ie: 2 , 4,4-trimethylpentene) 0.260mL (1.667mmol) and internal standard dodecane 0.095mL (0.4168mmol), the reaction progress was monitored by gas chromatography, and the reaction time was 5h. After the reaction was finished, the reaction was quenched with 3mol / L ammonia water, extracted with diethyl ether, the obtained organic layer was concentrated, and separated with a chromatographic silica gel column (300-400 mesh coarse-pore silica gel), and the eluent was diethyl ether:petroleum ether =1:50, finally obtained light yellow liquid, the total yield of chromatogram was 71%, diastereomer ratio cis-D 1 :trans-D 1 =1.2:1 (chromatographic integration rat...
Embodiment 2
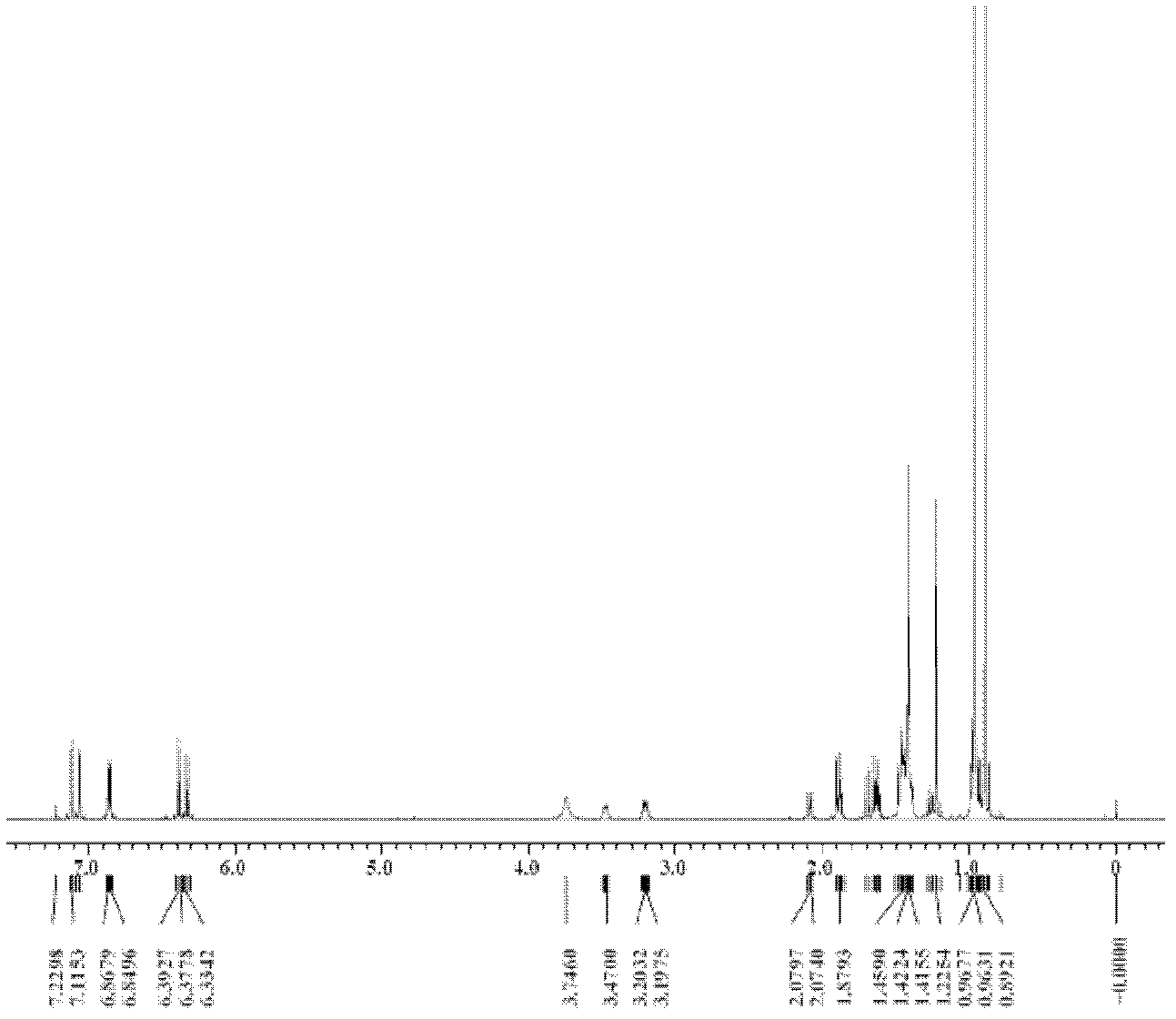
[0065] Embodiment 2: 4-methyl-2-propyl group-4-neopentyl-6-chloro-1,2,3,4-tetrahydroquinoline (cis-D 2 +trans-D 2 )
[0066] SnCl 2 0.3204g (1.6899mmol) and KMnO 4 Dissolve 0.1068g (0.676mmol) in 13mL of chloroform, add a small amount of hydrochloric acid dropwise, stir at room temperature for 5h, add n-butyraldehyde 0.152mL (1.6899mmol), p-chloroaniline 0.1078g (0.8450mmol), diisobutene 0.263 mL (1.6899mmol) and internal standard dodecane 0.096mL (0.4225mmol), the reaction progress was monitored by gas chromatography, and the reaction time was 36h. After the reaction was finished, the reaction was quenched with 3mol / L ammonia water, extracted with diethyl ether, the obtained organic layer was concentrated, and separated using a chromatographic silica gel column. The eluent was diethyl ether:petroleum ether=1:20, and finally a light yellow Liquid, target product chromatographic overall yield 45%, diastereomer ratio cis-D 2 :trans-D 2 =1:1.
[0067] Product Characterizat...
Embodiment 3
[0071] Embodiment 3: Preparation of 4-methyl-2-propyl-4-neopentyl-6-methoxy-1,2,3,4-tetrahydroquinoline (cis-D 3 +trans-D 3 )
[0072] SnCl 2 0.4394g (2.3175mmol) and FeCl 3 Dissolve 0.7518g (4.635mmol) in 15mL of 1,2-dichloroethane, stir at 0°C for 1h, raise the temperature to room temperature (30°C), add 0.209mL (2.3175mmol) of n-butyraldehyde, p-formaldehyde Oxyaniline 0.4335g (3.476mmol), diisobutylene 0.361mL (2.3175mmol) and internal standard dodecane 0.132mL (0.5794mmol), the reaction progress was monitored by gas chromatography, and the reaction time was 8h. After the reaction was finished, the reaction was quenched with 3mol / L ammonia water, extracted with ether, and the obtained organic layer was concentrated and separated with a chromatographic silica gel column, and the eluent was ethyl acetate:petroleum ether=1:20 to obtain light Yellow viscous liquid, the total yield of chromatography is 85%, the total yield of separation is 73%, the ratio of diastereoisomers...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com