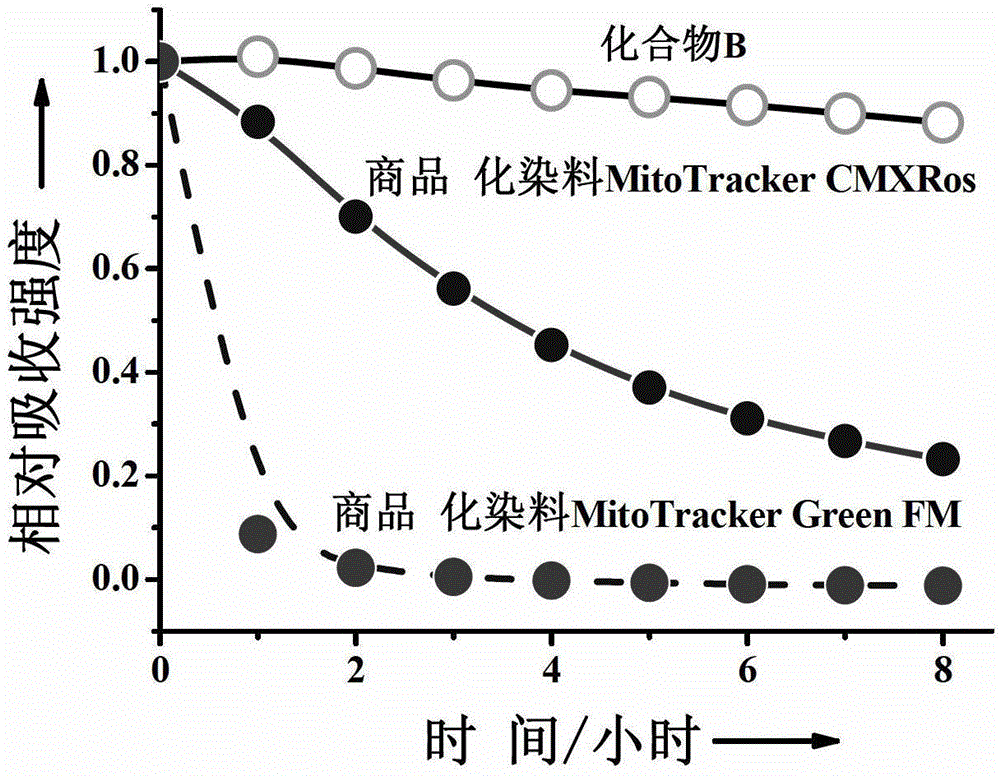
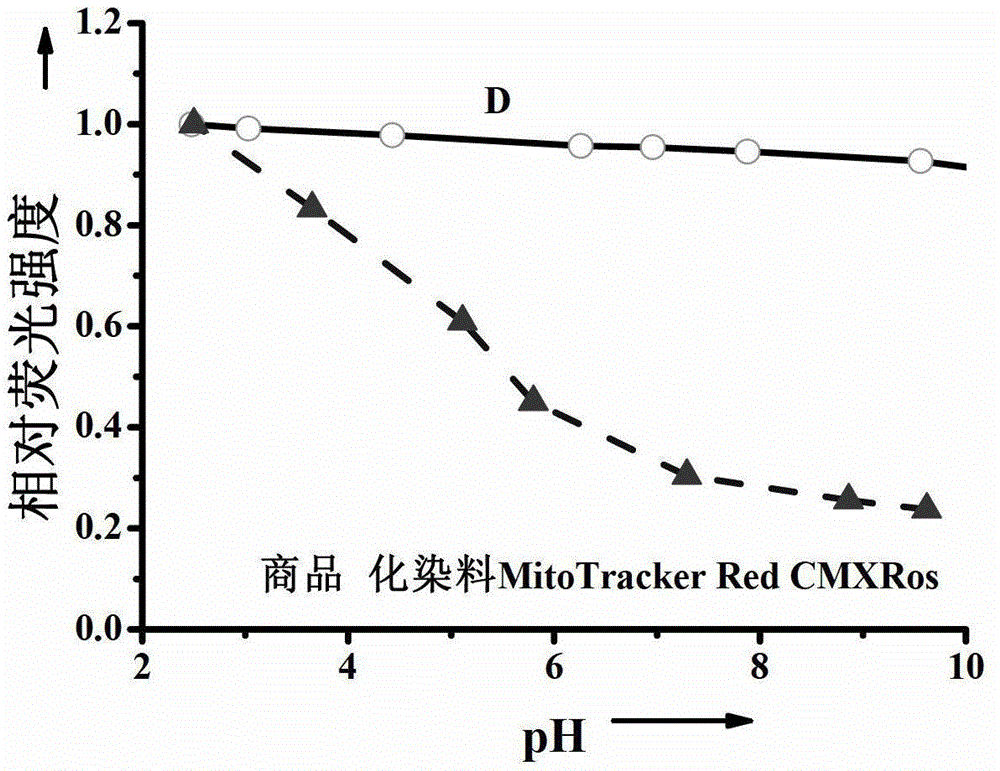
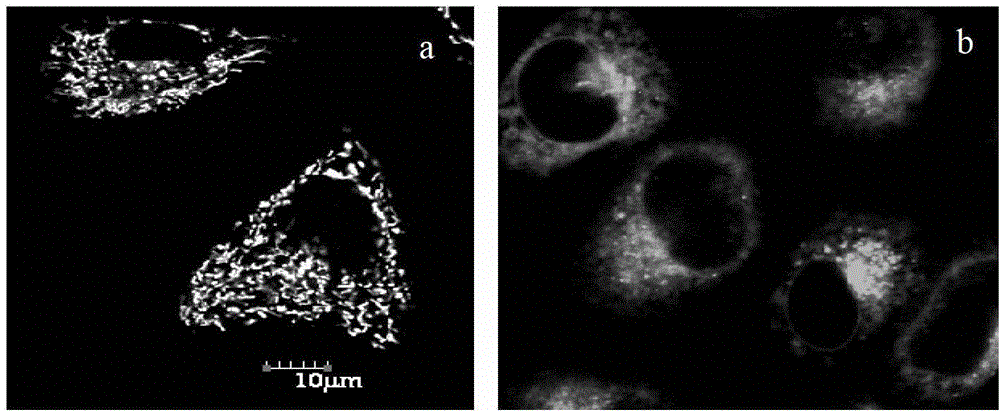
Single charge boron fluroride complexing dipyrrole methenyl fluorochrome and application thereof
A technology of dipyrrole methine and fluorescent dye, applied in methine/polymethine-based dyes, organic dyes, luminescent materials, etc., can solve the problems that the spectrum is easily affected by environmental factors of pH, the influence of fluorescence intensity, poor stability, etc., Achieving the effect of great potential application value, good resistance to pH interference and high photostability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Preparation of Intermediate A
[0052]
[0053] In a 250mL single-necked round-bottom flask, add 150mL of freshly steamed DCM, and replace with nitrogen several times under reduced pressure to remove the dissolved air in the solvent; then weigh 428mg (4mmol) of pyridine-2-carbaldehyde, and make it under stirring Dissolve, then inject 0.75mL (8mmol) 2,4-dimethylpyrrole with a syringe, quickly add a few drops of trifluoroacetic acid to catalyze, the whole system is carried out under nitrogen atmosphere, and rapid magnetic stirring, overnight. The mother liquor was rotatably evaporated to remove 100mL of solvent, and then 40mL of DCM solution containing 908mg (4mmol) 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ) was added dropwise under stirring within half an hour, and the color of the mother liquor changed from red to Brown to dark brown, continue to stir and gradually add 8 mL of anhydrous triethylamine dropwise. After the white smoke above the reaction solution disappe...
Embodiment 2
[0055] Preparation of Compound B
[0056]
[0057] Add A (0.65g, 2mmol) into a 100ml single-necked round bottom flask, dissolve it in 40ml of dried acetonitrile, add ethyl iodide (3.12g, 20mmol) dropwise into the reaction solution, protect it under nitrogen, and heat to reflux for 5h. The reaction solution was cooled to room temperature, the solvent was evaporated by rotary evaporation, and separated by neutral alumina column chromatography, the eluent was dichloromethane / methanol=50:1 (v / v), and finally an orange-red powder product was obtained with a yield of 58 %. 1 H NMR (400MHz,D 2 O)δ9.23(d,J=5.9Hz,1H),8.73(t,J=8.0Hz,1H),8.32(t,J=6.9Hz,2H),6.29(s,2H),4.68(q ,J=7.5Hz,2H),2.51(s,6H),1.50(t,J=7.3Hz,3H),1.35(s,6H); HR-TOF-MS C 20 h 23 BN 3 f 2 m / z 354.1945.
Embodiment 3
[0059] Synthesis of Intermediate C
[0060]
[0061] Compound A (0.65g, 2mmol) and benzaldehyde (2.2mmol) were charged into a 100mL single-necked bottle, and dried toluene (40mL), catalyst piperidine (1mL), and 4 Three molecular sieves were added to it, heated to reflux, TLC was used to monitor the reaction end point, silica gel column chromatography was used for separation and purification, the eluent was petroleum ether / ethyl acetate=5:1, and a deep red solid was obtained with a yield of 20%. 1 H NMR (400MHz, CDCl 3 )δ8.81(d,J=6.1Hz,1H),7.90(t,J=7.8Hz,1H),7.7(m,2H),7.50(m,2H),7.28-7.48(m,5H), 6.60(s,1H), 5.99(s,1H), 2.56(s,3H), 1.37(s,3H), 1.33(s,3H). TOF-LD C 25 h 22 BN 3 f 2 m / z 413.1863.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com