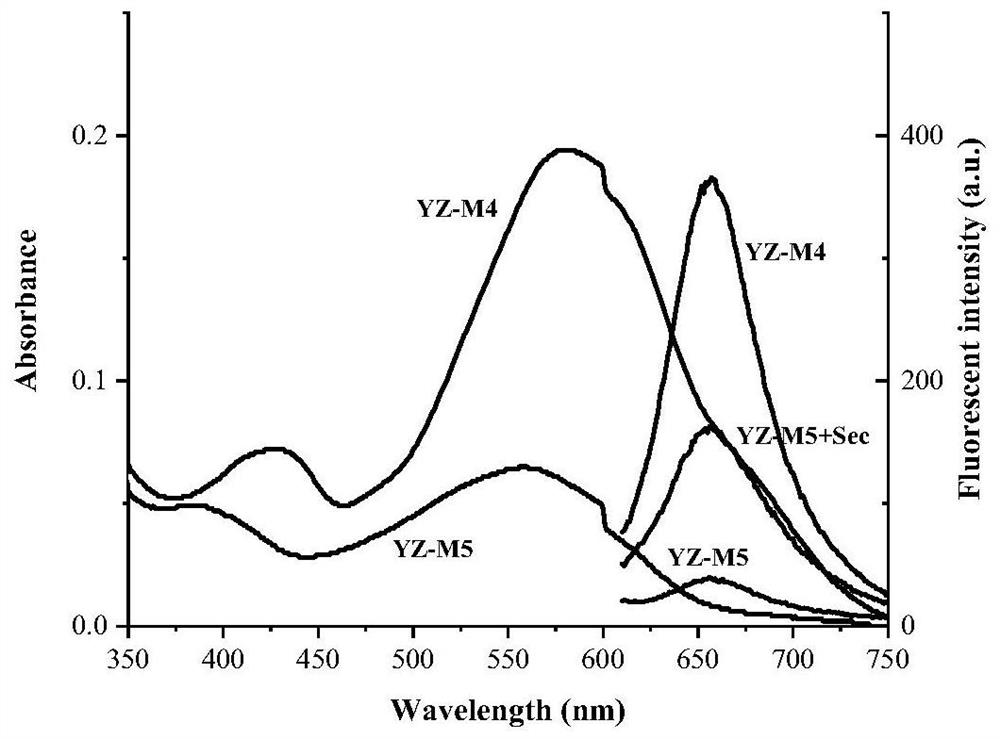
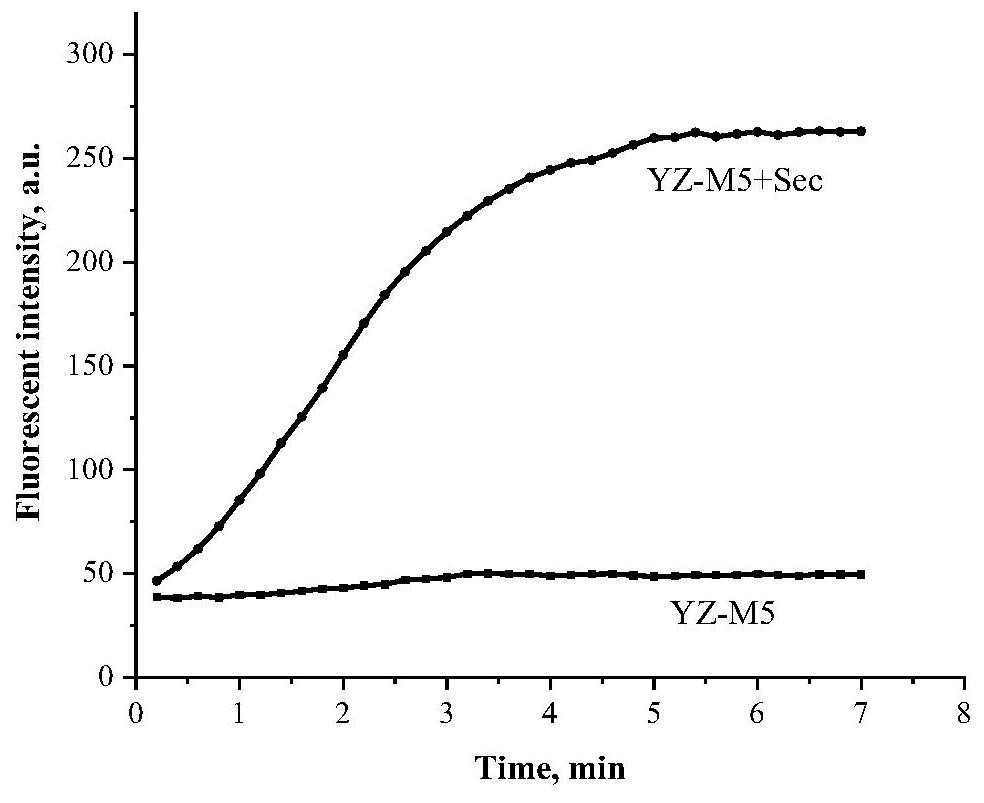
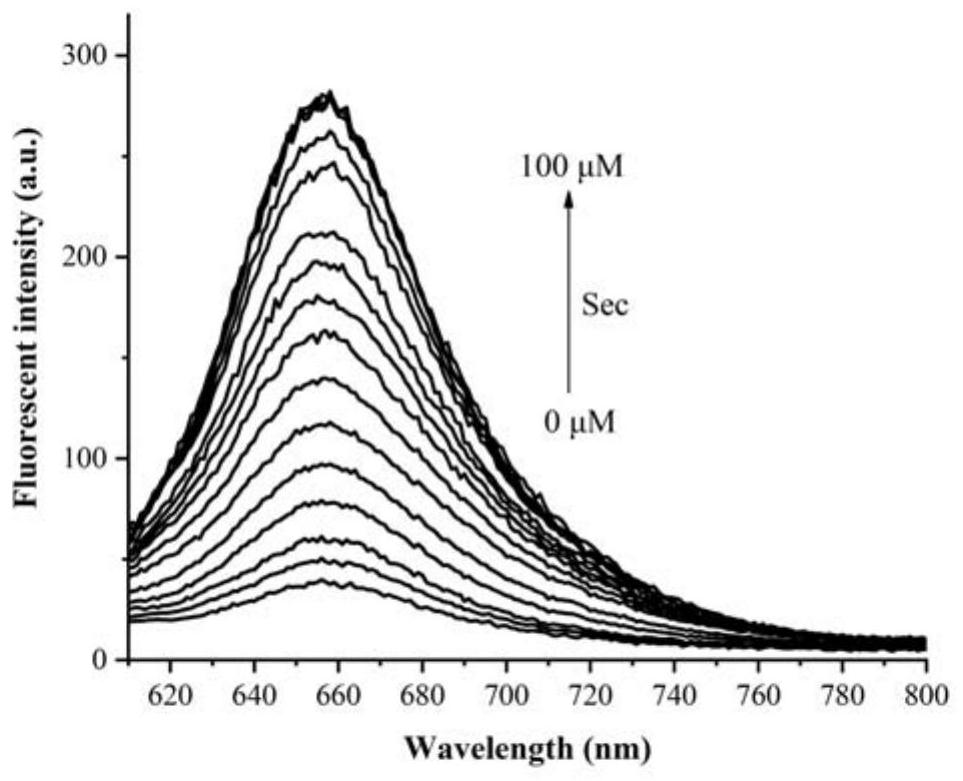
Near-infrared selenocysteine fluorescent probe as well as preparation method and application thereof
A technology of selenocysteine and fluorescent probes, which is applied in the field of near-infrared selenocysteine fluorescent probes and its preparation, can solve the problems affecting the sensitivity and accuracy of detection and analysis, unfavorable living body and deep tissue imaging, Weak penetrability and other problems, to achieve the effect of low detection limit, fast reaction speed and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of compound YZ-M3
[0031] In a 50 mL round bottom flask, add 15 mL concentrated H 2 SO 4 , and then in concentrated H 2 SO 4 Cyclohexanone (2.94 g, 0.03 mol) was added dropwise to the mixture, and after cooling to room temperature, 4-(diethylamino) salicylaldehyde (3.86 g, 0.02 mol) was added, and the reaction was vigorously stirred at 90° C. for 2.0 h. After the reaction was complete, it was cooled and poured into 200 mL of ice water, then 1.5 mL of 70% perchloric acid was added and allowed to stand overnight, a red precipitate precipitated, filtered, washed with cold water (50 mL×3), and dried. Obtained compound YZ-M3 red solid 5.05g, yield 71%.
Embodiment 2
[0033] Preparation of Compound YZ-M4
[0034] Compound YZ-M3 (711 mg, 2 mmol) and p-hydroxybenzaldehyde (366 mg, 3 mmol) were dissolved in acetic acid (20 mL). The reaction mixture was heated to 110°C for 2h. After the reaction was complete, the solvent was distilled off under reduced pressure. The crude product was purified by using silica gel column chromatography (DCM:MeOH=20:1, v / v) to obtain 598 mg of a black solid with a yield of 65%. 1 H NMR (400MHz,d6-DMSO)δ10.33(s,1H),8.48(s,1H),8.03(s,1H),7.87(d,J=9.4Hz,1H), 7.58(d,J= 8.6Hz, 2H), 7.43(dd, J=9.4, 1.9Hz, 1H), 7.27(s, 1H), 6.93(d, J=8.6Hz, 2H), 3.70(q, J=7Hz, 4H), 2.87(dd,J=18.3,13.0Hz,4H),1.89-1.77(m,2H),1.26(t,J=7Hz,6H). 13 C NMR(100MHz,d6-DMSO)δ191.42,163.49,160.15,158.85,156.04,148.68,137.56, 133.97,132.55,132.29,126.90,125.49,123.43,118.80,118.45,116.43,116.29,95.85,45.95,27.23,21.48 , 13.01. HRMS (C 24 h 26 NO 2 ) + :360.1956, calcd for (C 24 h 26 NO 2 ) + :360.1958.
Embodiment 3
[0036] Preparation of fluorescent probe YZ-M5
[0037]Add compound YZ-M4 (230mg, 0.5mmol) and anhydrous potassium carbonate (138mg, 1mmol) into DCM (10 mL) solution, cool down to 0°C, then add 2,4-dinitrobenzenesulfonyl chloride (192mg, 0.75 mmol) was dissolved in 10 mL of anhydrous DCM, and added dropwise to the above reaction solution. After the reaction was complete, the solvent was distilled off under reduced pressure. The crude product was purified by silica gel column chromatography (DCM:MeOH=30:1, v / v) to obtain 234 mg of fluorescent probe YZ-M5 as a black solid, with a yield of 68%. 1 H NMR (400MHz, d6-DMSO) δ9.14 (d, J = 2Hz, 1H), 8.64 (dd, J = 9, 2Hz, 1H), 8.58 (s, 1H), 8.32 (d, J = 9Hz, 1H),8.03(s,1H),7.94(d,J=9Hz,1H),7.70(d,J=9Hz,2H),7.53(dd,J=9,2Hz,1H), 7.35(d,J =9Hz, 2H), 7.30(d, J=2Hz, 1H), 3.73(q, J=7Hz, 4H), 2.89(t, J=6Hz, 4H), 1.89– 1.80(m, 2H), 1.26( t,J=7Hz,6H). 13 C NMR(100MHz,d6-DMSO)δ161.23,159.18,156.80, 152.05,149.38,148.89,148.60,135.68,134.04,13...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com