Genetically engineered bacterium of colon bacillus for producing arabinoside-cytidine monophosphate lipoid A, and application thereof
A technology of genetically engineered bacteria and monophosphoric acid lipids, applied in the field of genetically engineered bacteria, can solve problems affecting product quality and yield, numerous steps, and difficult operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1 Construction of mutant E.coli W3110 △lacl
[0018] 1. Obtaining the lacI gene knockout fragment
[0019] The lacI gene knockout fragment is obtained by chemical total synthesis or PCR step-by-step amplification, and its two ends are the upstream and downstream homology arms of the lacI gene, and the middle is a kan fragment. The nucleotide sequence of the lacI gene knockout fragment is shown in SEQ ID NO.1. The lacI gene knockout fragments XhoI and PstI were cloned into pBlueScript II SK(+) to obtain the recombinant plasmid pBlueScript II SK(+)-lacI(U)-pkan-lacI(D). Among them, there are loxP sites on both sides of the kan gene.
[0020] 2. Preparation and electrotransformation of knockout competent cells
[0021] Inoculate with Red recombinant helper plasmid pKD46 (Datsenko K A, Wanner B L. One-Step inactivation of chromosome genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA, 2000, 97(12): 6640-6645) coli W3110 (ATCC39936) was cult...
Embodiment 2
[0027] Construction of embodiment 2 mutant strain HW002
[0028] 1. Obtaining the knock-in fragment pagl-lpxE-pagP-Fkan
[0029] The PCR amplification method was used to obtain pagL, lpxE, pagP, and Fkan gene fragments, which were cloned into pWSK29 in turn to obtain the recombinant plasmid pWSK29-pagL-lpxE-pagP-Fkan. The knock-in fragment pagL-lpxE-pagP-Fkan with lacZ-α natural homology arm was amplified by PCR, and its nucleotide sequence is shown in SEQ IN NO.2. Among them, there are FRT sites on both sides of the kan gene.
[0030] 2. Preparation of knock-in competent cells and electrotransformation
[0031] The W3110△lacI strain carrying the pKD46 plasmid was used as the starting strain to prepare competent cells, and the method was the same as above. 500-1000ngpagL-lpxE-pagP-Fkan knock-in fragment was electrotransformed into competent cells, and the mutant strain W3110△lacI lacZ:: pagL-lpxE-pagP-Fkan.
[0032] 3. Removal of mutant resistance markers
[0033] By trans...
Embodiment 3
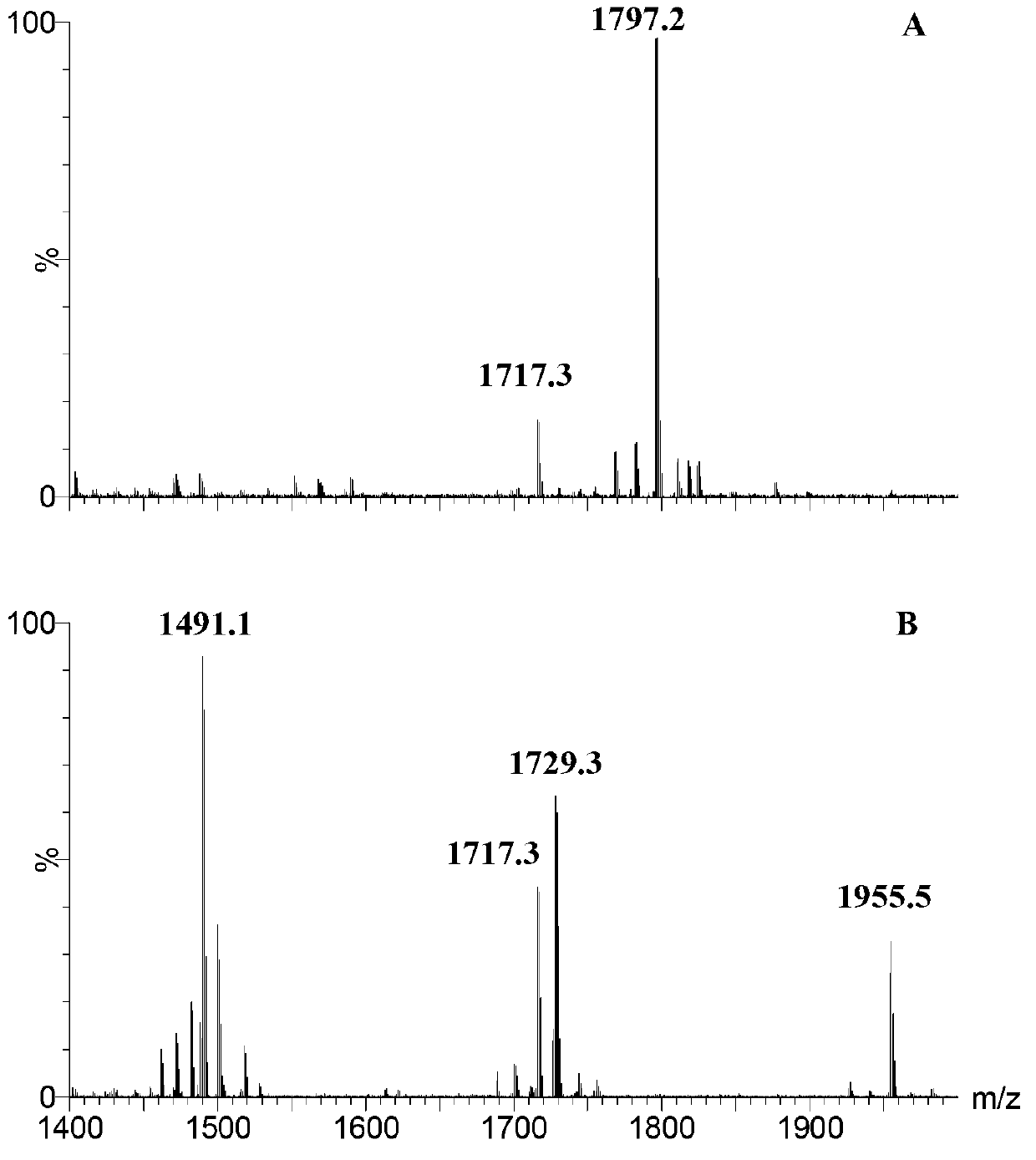
[0034] Lipid A structure analysis of embodiment 3 mutant strain HW002
[0035] 1. Lipid A extraction and thin layer chromatography (TLC) analysis of mutant strain HW002
[0036] Lipid A was extracted by chloroform / methanol / water mixed phase extraction. The overnight cultured bacterial solution was divided into initial OD 600 =0.02 transferred to 200mL LB liquid medium, cultured to OD at 37°C 600 = 1 hour 8000rpm centrifuge 10min to collect bacteria, ddH 2 After washing the cells once, use Bligh-Dyer one-phase system (chloroform / methanol / water, 1:2:0.8, v / v / v) to suspend the cells, magnetically stir for 1 hour, and centrifuge at 2000rpm for 20 minutes to separate the phases. Use a one-phase system Wash cell debris 2-3 times. Add 27mL of 12.5mM sodium acetate (PH4.5) solution, ultrasonically shake for 10min, and cleavage the sugar chains in a 100°C water bath for 30min. After cooling to room temperature, add 30mL chloroform and 30mL methanol to form a Bligh-Dyer two-phase s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com