Amylase mutant with improved heat stability and application thereof
An amylase and mutant technology, applied in the field of alkaline amylase mutants and their preparation, can solve problems such as limited application scope, and achieve the effects of broad application prospects, improved thermal stability, and efficient degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Analysis and method of amylase thermostability site-directed mutation
[0020] Through the analysis of amylase sequence and 3D space structure, several amino acid residues (Pro 58, His 199, Gln 216, Asn 292, Pro 467) related to the improvement of the thermal stability of the enzyme in the active center were determined.
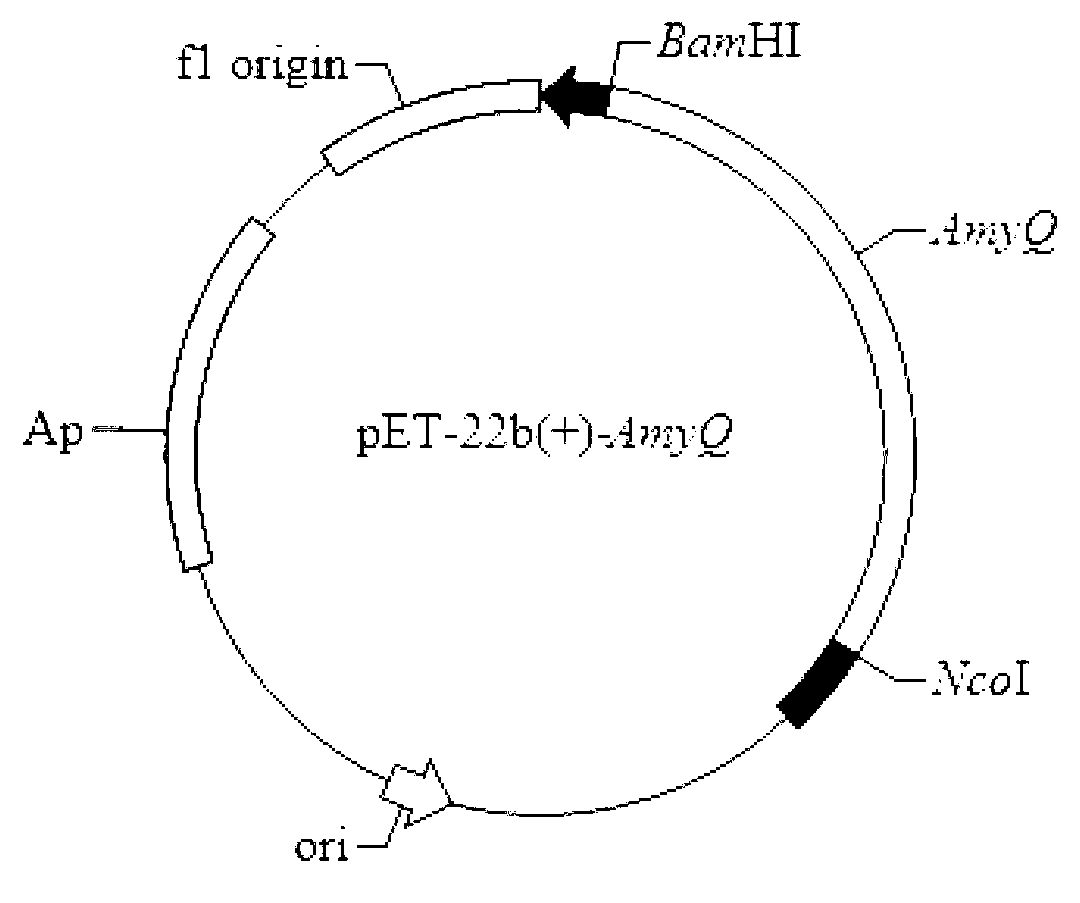
[0021] According to the amylase sequence of Bacillus alcalophilus, it was fully synthesized by chemical total synthesis, and then cloned into the plasmid pET-22b(+) to construct the recombinant plasmid pAmyQ.
[0022] For site-directed mutagenesis at different sites, design corresponding site-directed mutagenesis primers (Table 1). Using site-directed mutagenesis primers and recombinant plasmid pAmyQ, the amylase was subjected to site-directed mutagenesis. PCR enzyme was used to amplify the recombinant plasmid pAmyQ with mutant primers. The amplified fragments were recovered and purified using a gel recovery kit. The obtained purified frag...
Embodiment 2
[0023] Example 2: Analysis and method of amylase thermostability site-directed mutation
[0024] Determination of Alkaline Amylase Activity by DNS Method
[0025] 1. Preparation of DNS reagent: Weigh 3.25g of 3,5-dinitrosalicylic acid and dissolve it in a small amount of water, transfer it into a 500mL volumetric flask, add 162.5mL of 2mol / L sodium hydroxide solution, and then add 22.5g of glycerin, Shake well, dilute to 500mL, store in a brown bottle and place in a refrigerator at 4°C until use.
[0026] 2. Preparation of glucose standard curve: prepare glucose solutions with different concentrations from 0.2g / L to 1.0g / L. Take 1mL of different concentrations of glucose and mix it with the same volume of DNS solution, put it in a boiling water bath, and keep the water bath for 10min. Cool with cold water, dilute to 10mL, A 540 Measure the absorbance. Take the concentration of glucose as the abscissa and the absorbance as the ordinate to make a standard curve.
[0027] 3....
Embodiment 3
[0033] Embodiment 3: the thermostability determination analysis of amylase at 50 DEG C
[0034] Through measurement, it was found that the half-life of single mutants P58A, H199L, Q216V, N292W and P467V at 50°C (Table 2) were all improved, among which Q216V had the most significant effect, and the half-life was doubled. On this basis, H199L, Q216V, and P467V were preferably combined with mutations to obtain four mutants, H199L / Q216V, H199V / P467V, Q216V / P467V, and H199L / Q216V / P467V. It was found by measurement that their half-lives at 50°C (Table 1) 3) All have been improved, among which the H199L / Q216V / P467 triple mutant has the most significant effect, and the half-life is increased to 2.9 times of the original. The amylase has strong thermal stability under alkaline conditions.
[0035] Table 3 The thermostability of preferred site combination mutant recombinase at 50°C
[0036]
[0037]
[0038]
[0039]
[0040]
[0041]
[0042]
[0043]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com