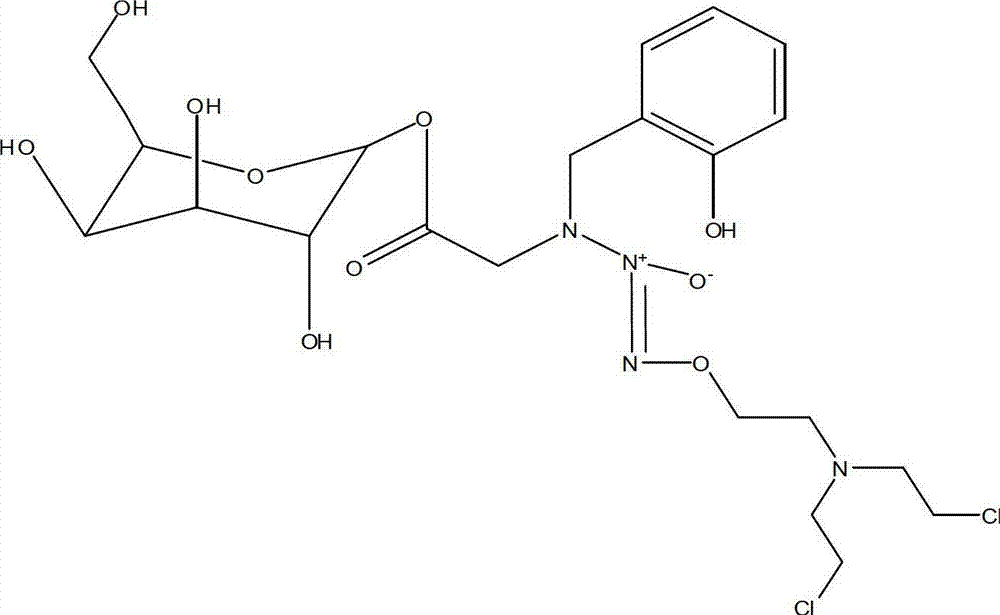
Pharmaceutical composition containing beta-galactosylation azo ene glycol and preparation method thereof
A technology for the alkylation of azodiol and galactose, which is applied in the preparation of sugar derivatives, esterified saccharides, chemical instruments and methods, etc., can solve the problems of accelerated degradation, environmental temperature and humidity sensitivity, and organs that do not reach the drug Dosage and other issues, to achieve the effect of prolonging the release time and good tissue specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Combination medicine components: 7g β-galactosylated azoene diol, 5g Xenopuscin, 70g sodium montmorillonite, 18g monostearoyl polyglyceride with a degree of polymerization of 6-14.
[0030] Combination medicine preparation:
[0031] 1) Dissolve 8g of Xenopus in 20ml of ethanol aqueous solution, the volume ratio of ethanol to water is 1:4, then add 70g of montmorillonite, stir, make Xenopuscin fully adsorbed in the montmorillonite, filter, Take the montmorillonite, evaporate the alcohol, and put it in an oven for drying;
[0032] 2) Dissolve 10g of β-galactosylated azoene glycol in 25ml of ethanol, and then add the smectite obtained in step 1) with luteolin adsorbed and stir to make the NO donor drug fully adsorbed on In the montmorillonite, filter, take the montmorillonite, and evaporate the alcohol;
[0033] 3) Add 18 g of monostearoyl polyglycerol ester to the smectite obtained in step 2) with the adsorbed Xenopus and NO donor drug, and mix well to obtain β-galactosylated az...
Embodiment 2
[0035] Combination medicine components: 12g β-galactosylated azoene glycol, 7g xenopuscin, 60g sodium montmorillonite, 21g polydimethylsiloxane with specific gravity of 0.96-0.97.
[0036] Combination medicine preparation:
[0037] 1) Dissolve 10g of Xenopus in 35ml of ethanol aqueous solution, the volume ratio of ethanol to water is 3:7, then add 60g of montmorillonite, stir to make Xenopusin fully absorbed in the montmorillonite, filter, Take the montmorillonite, evaporate the alcohol, and put it in an oven for drying;
[0038] 2) Dissolve 16g of β-galactosylated azoene diol in 30ml of ethanol, and then add the smectite obtained in step 1) with luteolin adsorbed, and stir to make the NO donor drug fully adsorbed on In the montmorillonite, filter, take the montmorillonite, and evaporate the alcohol;
[0039] 3) Add 21 g of stearoyl polyglycerol ester to the montmorillonite adsorbed in step 2) with Xenopus and NO donor drug, and mix well to obtain β-galactosylated azoene Combination...
Embodiment 3
[0041] Combination medicine components: 30g β-galactosylated azoene glycol, 4g xenopuscin, 50g calcium montmorillonite, 16g ethyl cellulose with a molecular weight of 2000.
[0042] Combination medicine preparation:
[0043] 1) Dissolve 6g of Xenopus in 30ml of ethanol aqueous solution, the volume ratio of ethanol to water is 1:4, then add 50g of calcium montmorillonite, stir to make Xenopuscin fully adsorbed in the montmorillonite, filter , Take the montmorillonite, evaporate the alcohol, and put it in an oven for drying;
[0044] 2) Dissolve 30g of β-galactosylated azoene diol in 80ml of ethanol, and then add the smectite obtained in step 1) to adsorb luteolin, and stir to make the NO donor drug fully adsorbed on In the montmorillonite, filter, take the montmorillonite, and evaporate the alcohol;
[0045] 3) Add 16 g of ethyl cellulose to the montmorillonite that has adsorbed xenopus and NO donor drug obtained in step 2), and mix well to obtain β-galactosylated azoene glycol Of co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com