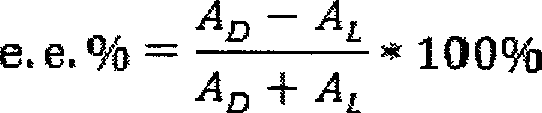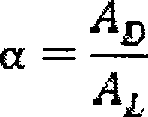Flat sheet membrane for chiral separation of amino acid and preparation method thereof
A chiral separation, flat membrane technology, applied in semi-permeable membrane separation, cyanide reaction preparation, chemical instruments and methods, etc. To achieve the effect of selective and efficient adsorption and desorption, efficient selective adsorption and desorption, and improved desorption efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Take a three-necked flask device, add 50ml of THF that has been treated with water removal to condense and reflux, and add NIPAAm monomer to the three-necked flask to make the NIPAAm concentration 0.1mol / L. After adding the chiral monomer AAc-L-PheEt and 0.10 g of the cross-linking agent MBAA according to the mass ratio r of 0.2, stir at 30 ° C for 30 minutes, slowly raise the temperature to 50 ° C, stabilize for 20 minutes, add 0.04 g to initiate Azobisisobutyronitrile (AIBN) was used to react at constant temperature for 6 hours. After the reaction, petroleum ether was added to the reaction solution to precipitate, and microgel P(NIPAAm-co-AAc-L-PheEt) was obtained by suction filtration. Store in a cool place.
[0037] (2) Synthesis of temperature-sensitive film-forming substances: Take 10 g of alkali-treated PVDF powder and add it to a three-necked flask, pass nitrogen gas, add solvent N, N-dimethylformamide (DMF) with 6 times the mass of PVDF, and put it in a wat...
Embodiment 2
[0041] (1) Take a three-necked flask device, add 50ml of THF that has been treated with water removal to condense and reflux, and add NIPAAm monomer to the three-necked flask to make the NIPAAm concentration 0.13mol / L. After adding the chiral monomer AAc-L-PheEt and 0.20g of the cross-linking agent MBAA according to the mass ratio r of 0.2, stir at 30°C under nitrogen for 60 minutes, slowly raise the temperature to 70°C, stabilize for 40 minutes, add 0.08g to trigger Azobisisobutyronitrile (AIBN) was used to react at constant temperature for 10 hours. After the reaction, petroleum ether was added to the reaction solution to precipitate, and microgel P (NIPAAm-co-AAc-L-PheEt) was obtained by suction filtration. Store in a cool place.
[0042] (2) Synthesis of temperature-sensitive film-forming substances: Take 10 g of alkali-treated PVDF powder and add it to a three-necked flask, pass nitrogen gas, add solvent N, N-dimethylformamide (DMF) with 12 times the mass of PVDF, and hea...
Embodiment 3
[0046] (1) Take a three-necked flask device, add 50ml of THF that has been treated with water removal to condense and reflux, and add NIPAAm monomer to the three-necked flask to make the NIPAAm concentration 0.15mol / L. After adding the chiral monomer AAc-L-PheEt and 0.1g of the cross-linking agent MBAA according to the mass ratio r of 0.2, stir at 30°C under nitrogen for 60 minutes, slowly raise the temperature to 50°C, stabilize for 30 minutes, add 0.05g to trigger Reagent azobisisobutyronitrile (AIBN), constant temperature reaction for 10 hours. After the reaction was completed, petroleum ether was added to the reaction liquid to precipitate, and the microgel P(NIPAAm-co-AAc-L-PheEt) was obtained by suction filtration, which was dried and stored in a cool place.
[0047] (2) Synthesis of temperature-sensitive film-forming substances: Take 10 g of alkali-treated PVDF powder and add it to a three-necked flask, pass nitrogen gas, add solvent N, N-dimethylformamide (DMF) with 12...
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com