Production method of iron phosphate nano powder body with controllable size and granularity
A technology of nano powder and production method, which is applied in the direction of nanotechnology, nanotechnology, nanotechnology, etc. for materials and surface science, and can solve the problem of difficult control of material purity, different sizes of iron phosphate particles, and batches of iron phosphate Poor stability and other problems, to achieve the effect of reflecting low requirements, high product utilization rate and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
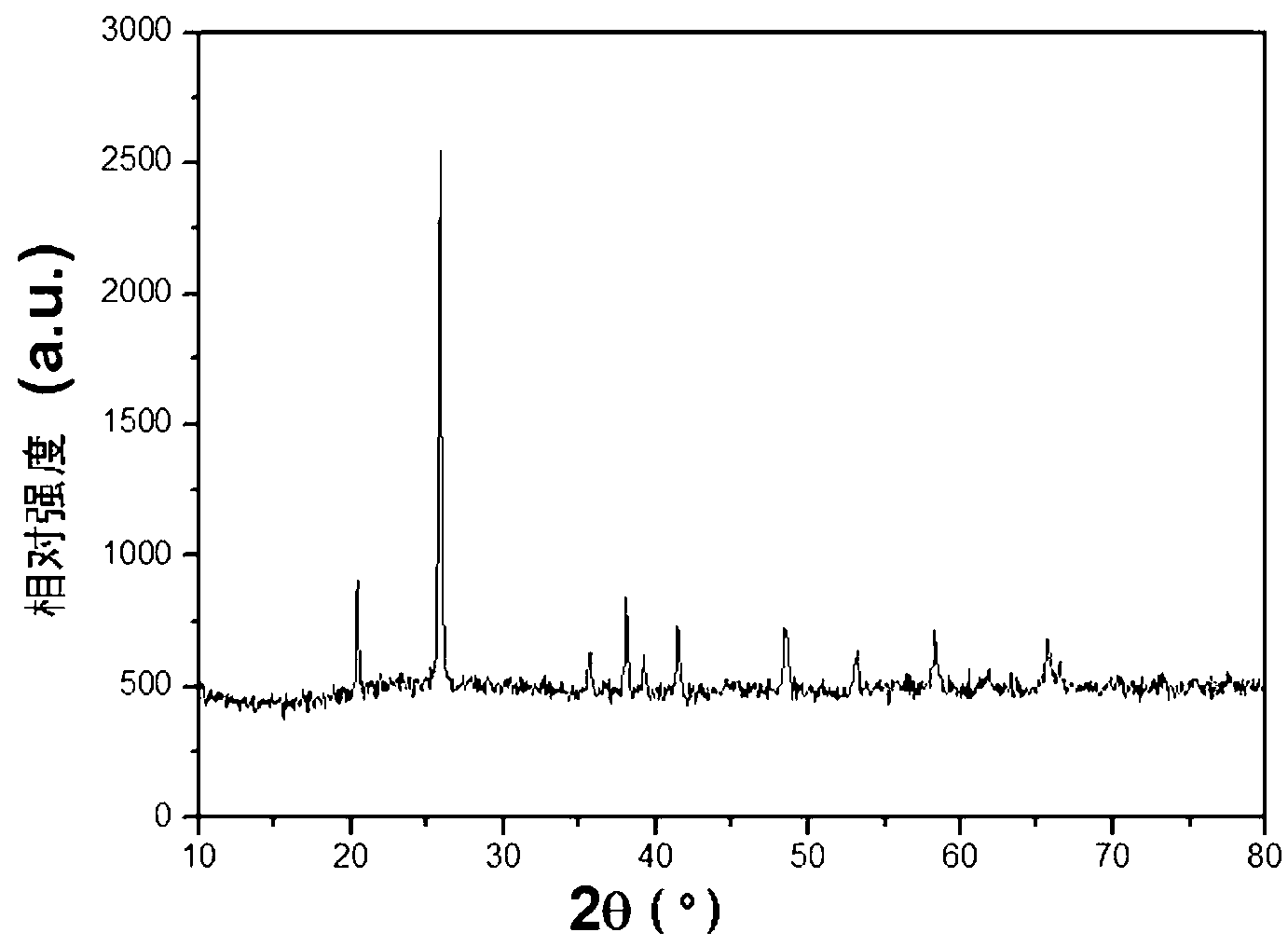
Embodiment 1
[0059] Take 75mL of ultrapure water to dissolve 0.4mol phosphoric acid and 0.1mol disodium hydrogen phosphate, adjust the pH of the solution to 4.0 with 5M sodium hydroxide and concentrated phosphoric acid solution, and finally obtain 100mL of 5mol / L phosphate base solution A 1 . Prepare 2mol / L ferric nitrate solution B 1 .
[0060] 100mL A 1 Place in a three-necked bottle and heat at a constant temperature of 90°C in an oil bath. After the temperature of the solution in the three-necked bottle is stable, quickly dissolve 150mL 1 , 2g of glucose, and 1g of tapioca starch were added to A 1 , mechanically stirred evenly, then slowly added 100mL B at 10mL / min 1 solution, adjust the pH of the solution to 3.0. After the dropwise addition, the three-neck flask was stirred at a constant temperature of 90°C in a water bath for 60 minutes, and the obtained ferric phosphate precipitate was washed with deionized water and centrifuged 4 times. The precursor was dried at 120°C for 1 ...
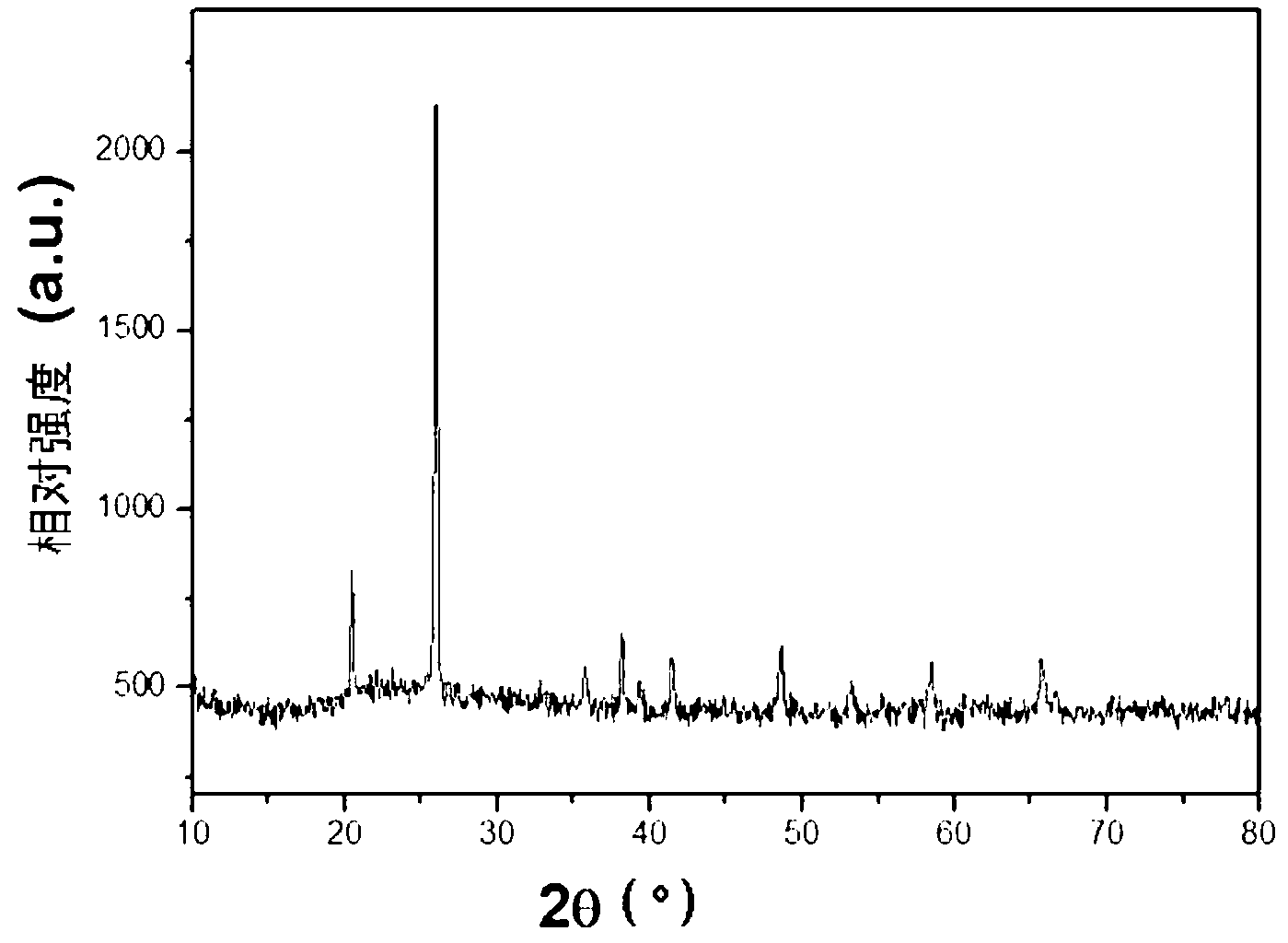
Embodiment 2
[0062] Mix ultrapure water and ethylene glycol at a ratio of 3:1, take 75mL of mixed solvent to dissolve 0.3mol phosphoric acid and 0.3mol disodium hydrogen phosphate, adjust the pH of the solution to 5.0 with 5M sodium hydroxide solution and concentrated phosphoric acid, and obtain 100mL of 6mol / L Phosphate base solution A 2 . Prepare 4mol / L ferric chloride aqueous solution B 2 .
[0063] 100mL A 2 Put it in a three-necked bottle and heat it in a 90°C oil bath. After the temperature of the solution in the three-necked bottle is stable, quickly add 100mL of B 2 Add to A together with 2g maltose and 1g SDBS 2 , mechanically stirred evenly, then slowly added 100mL B at 10mL / min 1 solution, adjust the pH of the solution to 3.0. Stir at a constant temperature of 90° C. for 120 minutes, and the obtained ferric phosphate precipitate is washed with deionized water and centrifuged 4 times. The precursor was dried at 120°C for 1 hour, and the resulting powder was subjected to a...
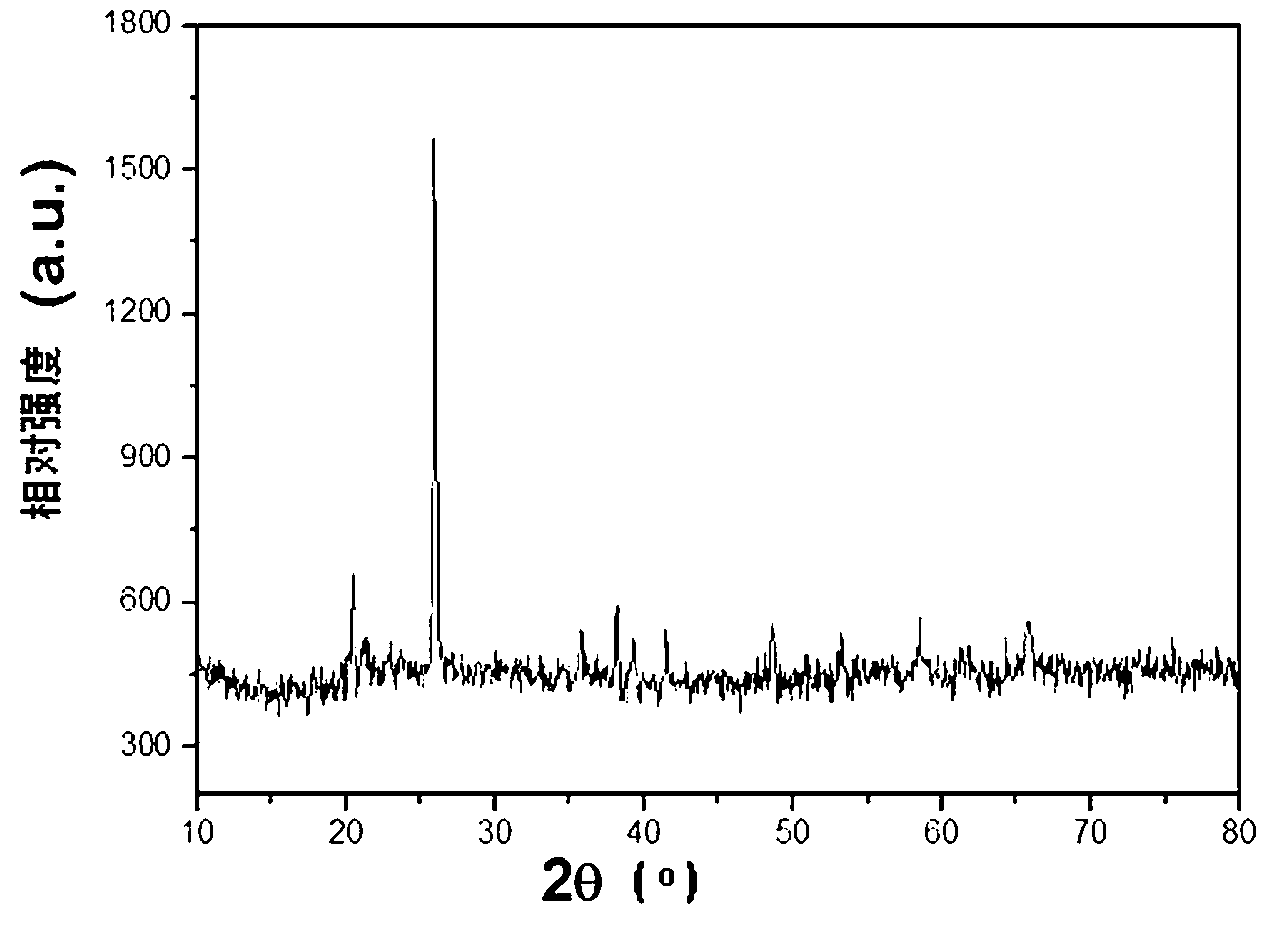
Embodiment 3
[0065] Mix ultrapure water and ethylene glycol at 3:1, take 80mL of mixed solvent to dissolve 0.2mol phosphoric acid and 0.4mol disodium hydrogen phosphate, adjust the pH of the solution to 5.0 with 5M sodium hydroxide solution and concentrated phosphoric acid, and obtain 100mL of 6mol / L Phosphate base solution A 3 . Prepare 3mol / L ferric nitrate aqueous solution B 3 .
[0066] 100mL A3 Put it in a three-necked bottle and heat it in a 90°C oil bath. After the temperature of the solution in the three-necked bottle is stable, quickly add 100mL of B 3 , 1g soluble starch, 1g fructose, 1g CTAB were added to A 3 , mechanically stirred evenly, then slowly added 50mL of B at a rate of 10mL / min 3 solution, adjust the pH of the solution to 3.5. Stir at constant temperature at 90° C. for 60 minutes, and the obtained precipitate is washed with deionized water and centrifuged 4 times. The precursor was dried at 120°C for 1 hour, and the obtained powder was subjected to constant tem...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com