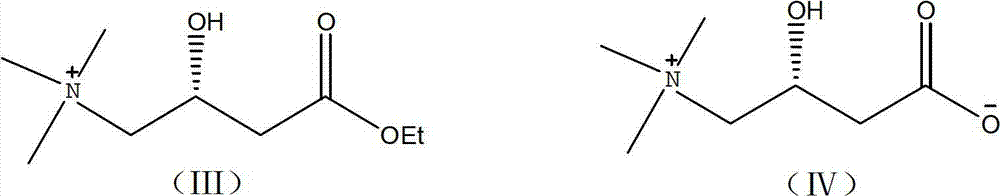L-carnitine preparation method
A technology of L-carnitine and organic bases, applied in the preparation of organic compounds, chemical instruments and methods, preparation of cyanide reactions, etc., can solve the requirements of turbidity, which are also very strict, the separation process is complicated, and the workload of enzyme screening is large. and other problems to achieve the effect of reducing difficulty, reducing production costs and reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
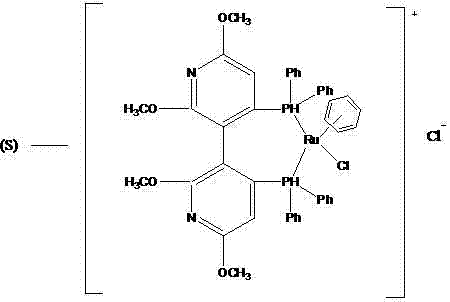
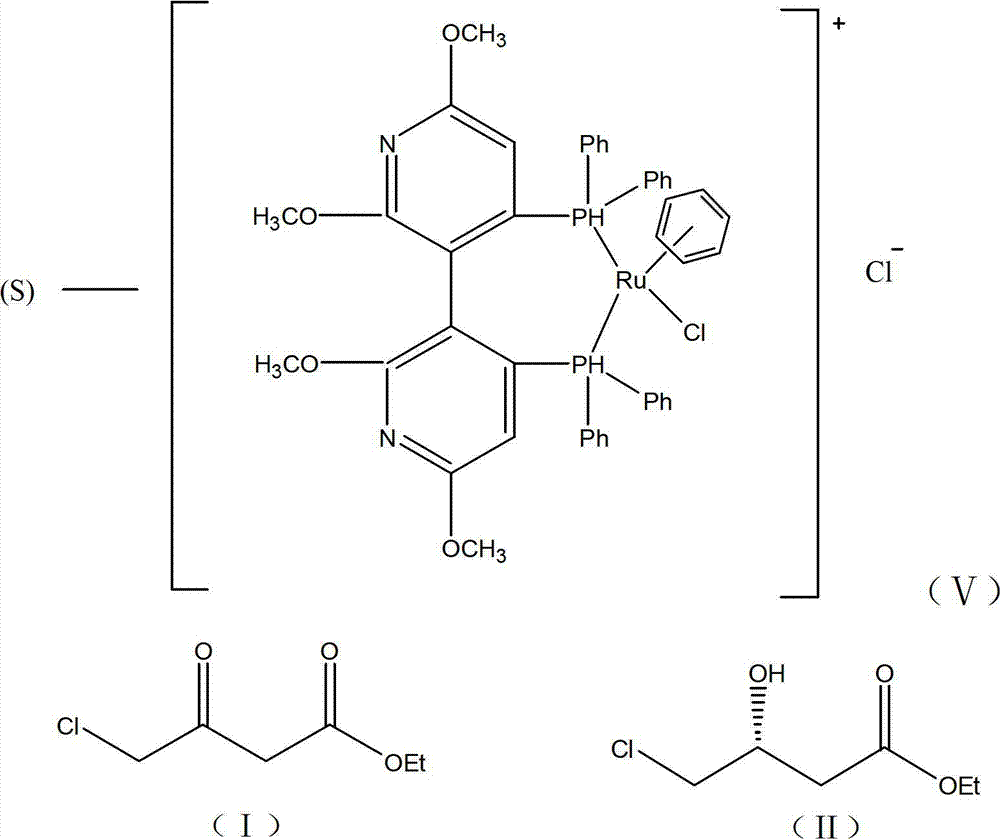
[0020] (1) Add 18000g (391.3mol) absolute ethanol in the glass-lined kettle of 100L cleaning and drying, 3000g (18.2mol) ethyl chloroacetoacetate, 100g deionized water, 1.6g formula (V) catalyst (present embodiment Among them, the molar ratio of ethyl chloroacetoacetate:catalyst=10000:1). Seal the reactor to N 2 Replace the air in the reactor, and then use H 2 Replacement of N in the reactor 2 , control H 2 The pressure is 6 atm, and the reaction kettle is placed in an oil bath at 100°C to stir the reaction. After asymmetric catalytic hydrogenation reaction for 6 hours, the ethanol reaction liquid containing (R)-4 chloro-3-hydroxy-butyl ethyl ester is obtained, cooled, and placed in the dark at a low temperature. Its ee value after testing is 96%; (2) the ethanolic solution of 1362g (20.02mol) sodium ethylate is dissolved in the ethanol of the 96% (R)-4 chloro-3 hydroxy-butyl ester ethyl ester that step (1) obtains In the reaction solution, the temperature was lowered to -...
Embodiment 2
[0022] (1) Add 18000g (391.3mol) dehydrated alcohol in 100L clean and dry glass-lined kettle, 3000g (18.2mol) ethyl chloroacetoacetate, 100g deionized water, 32g formula (V) catalyst (in this embodiment , ethyl chloroacetoacetate:catalyst molar ratio=500:1). Seal the reactor to N 2 Replace the air in the reactor, and then use H 2 Replacement of N in the reactor 2 , control H 2 The pressure is 1 atm, the reaction kettle is placed in an 80°C oil bath to stir the reaction, and after the asymmetric catalytic hydrogenation reaction for 12 hours, the ethanol reaction solution containing (R)-4 chloro-3-hydroxy-butyl ethyl ester is obtained, and it is left to cool, and it is kept at a low temperature and protected from light. , after detection its ee value is 92.95%; (2) the ethanolic solution of 1610.1g (23.66mol) sodium ethylate is dissolved in the 92.95% (R)-4 chloro-3 hydroxy-butyl ester ethyl ester that step (1) obtains In the ethanol reaction liquid, cool down to -10~-5°C, a...
Embodiment 3
[0024] (1) Add 18000g (391.3mol) absolute ethanol, 3000g (18.2mol) ethyl chloroacetoacetate, 100g deionized water, 0.4g formula (V) catalyst (chloroacetyl Ethyl acetate:catalyst=40000:1). Seal the reactor to N 2 Replace the air in the reactor, and then use H 2 Replacement of N in the reactor 2 , control H 2 The pressure is 20 atm, and the reaction kettle is placed in an oil bath at 30°C for 48 hours of stirring reaction, and the ethanol reaction liquid of (R)-4 chloro-3-hydroxy-butyl ester ethyl ester is obtained, which is left to cool, and placed in the dark at a low temperature. After testing, its ee value is 92%; (2) the ethanolic solution of 1857.7g (27.3mol) sodium ethylate is dissolved in the ethanol reaction solution of 92% (R)-4 chloro-3 hydroxyl-butyl ester ethyl ester that step (1) obtains, cooling to 10-15°C, add Me under stirring 3 N gas, Me 3 The molar ratio of N to (R)-4 chloro-3-hydroxy-butyl ethyl ester is 3:1, maintain the reaction temperature at 10-15°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com