Preparation method of atorvastatin calcium
A technology of atorvastatin calcium and intermediates, applied in the field of drug synthesis, can solve the problems of high environmental toxicity, increase and synthesis cost, expensive dosage, etc., and achieve the effect of good operation safety performance, low cost, and low reagent price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
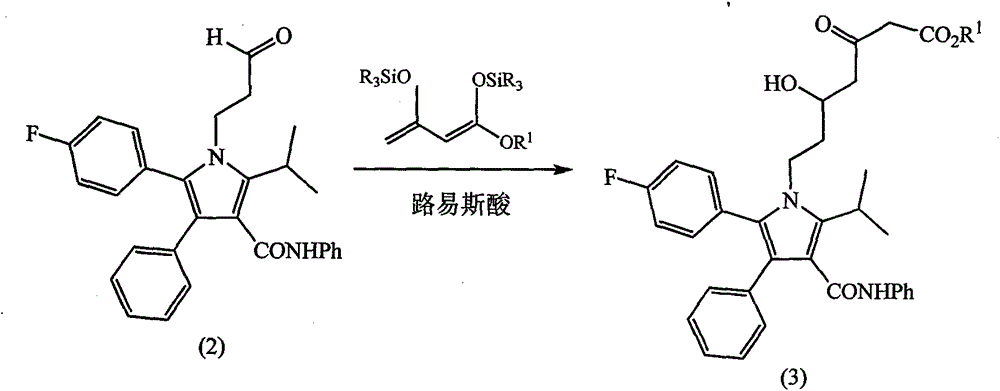
Examples
Embodiment 1
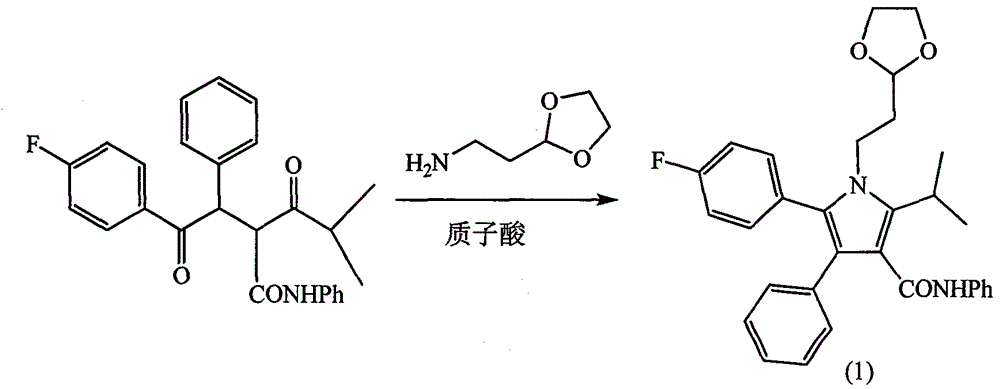
[0030] (1) Synthesis of intermediate (1)
[0031] Under the protection of nitrogen, 418 g (1 mol) of 5-methyl-2-phenyl-1-(4-fluorophenyl)-3-(phenylcarbamoyl)-1,4-hexanedione, 2-( 140g (1.2mol) of 2-aminoethyl)-1,3-dioxolane was added to 1000mL of tetrahydrofuran, the equivalent of pivalic acid was added dropwise, heated to reflux for 8h, the solvent was evaporated under reduced pressure, and the solid obtained was acetone -Ethyl acetate (1:1) was recrystallized to obtain 359 g of intermediate (1), with a yield of 72%. Characterization data:
[0032] m.p: 159-160°C. 1 H NMR(CDCl 3 , 400MHz) δ: 1.54 (d, 6H, J = 7Hz), 1.91 (m, 2H), 3.60 (sep, 1H, J = 7Hz), 3.7-4.1 (m, 6H), 4.74 (t, 1H, J = 4.3 Hz), 7.0-7.3 (m, 15H); LC-MS (m / z): 394 (M+1).
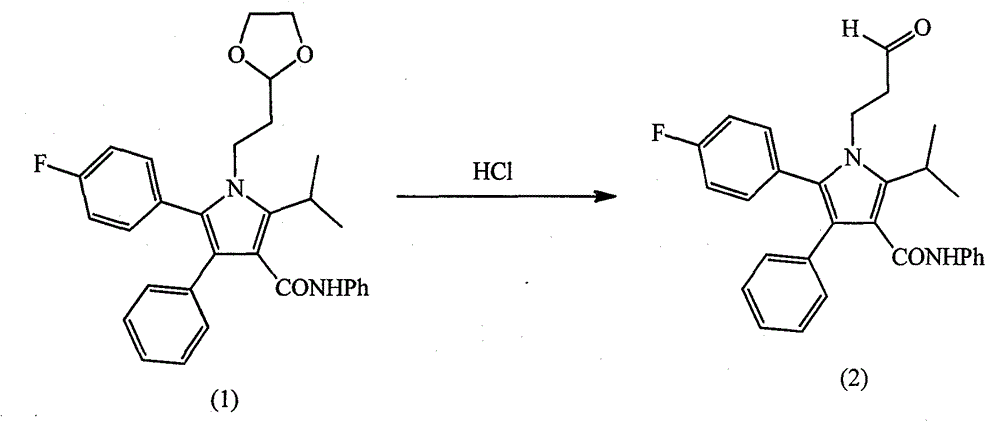
[0033] (2) Synthesis of intermediate (2)
[0034] Intermediate (1) 300g (0.6mol) was added to 1000mL methanol, 50mL 37% concentrated hydrochloric acid was added dropwise, stirred at room temperature for 3h, 0~5℃ was added dropwise 20% NaOH solutio...
Embodiment 2
[0046] (1) Synthesis of intermediate (1)
[0047] Under the protection of nitrogen, 418 g (1 mol) of 5-methyl-2-phenyl-1-(4-fluorophenyl)-3-(phenylcarbamoyl)-1,4-hexanedione, 2-( 140g (1.2mol) of 2-aminoethyl)-1,3-dioxolane was added to 1000mL of methyl tert-butyl ether, the equivalent of acetic acid was added dropwise, heated to reflux for 8h, and the solvent was evaporated under reduced pressure. The solid was recrystallized with acetone-ethyl acetate (1:1) to obtain intermediate (1) with a yield of 70%.
[0048] (2) Synthesis of intermediate (2)
[0049] Intermediate (1) 300g (0.6mol) was added to 1000mL methanol, 50mL 37% concentrated hydrochloric acid was added dropwise, stirred at room temperature for 3h, 0~5℃ was added dropwise 20% NaOH solution to adjust pH 6~7, 1000mL of formazan was added with stirring Butyl tert-butyl ether, let stand overnight, a large amount of solid precipitated, filtered, washed, and dried under vacuum to obtain intermediate (2) (3-[2-(4-fluorophenyl...
Embodiment 3
[0060] (1) Synthesis of intermediate (1)
[0061] Under the protection of nitrogen, 418 g (1 mol) of 5-methyl-2-phenyl-1-(4-fluorophenyl)-3-(phenylcarbamoyl)-1,4-hexanedione, 2-( 140g (1.2mol) of 2-aminoethyl)-1,3-dioxolane was added to 1000mL of dioxane, and the equivalent of formic acid (or propionic acid, butyric acid) was added dropwise, heated to reflux for 8h, and reduced pressure The solvent was evaporated, and the obtained solid was recrystallized with acetone-ethyl acetate (1:1) to obtain intermediate (1) with a yield of 73%.
[0062] (2) Synthesis of intermediate (2)
[0063] Intermediate (1) 300g (0.6mol) was added to 1000mL methanol, 50mL 37% concentrated hydrochloric acid was added dropwise, stirred at room temperature for 3h, 0~5℃ was added dropwise 20% NaOH solution to adjust pH 6~7, 1000mL of formazan was added with stirring Butyl tert-butyl ether, let stand overnight, a large amount of solid precipitated, filtered, washed, and dried under vacuum to obtain intermedi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com