Solid-phase synthesis method of antibacterial peptide Iseganan
A solid-phase synthesis, Esgenan technology, applied in the preparation methods of peptides, chemical instruments and methods, peptides, etc., can solve the problem of unable to meet the requirements of large-scale industrial production, unable to ensure the correct pairing of disulfide bonds, increase after Deal with problems such as difficulty, and achieve the effects of high yield, low pollution and few by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
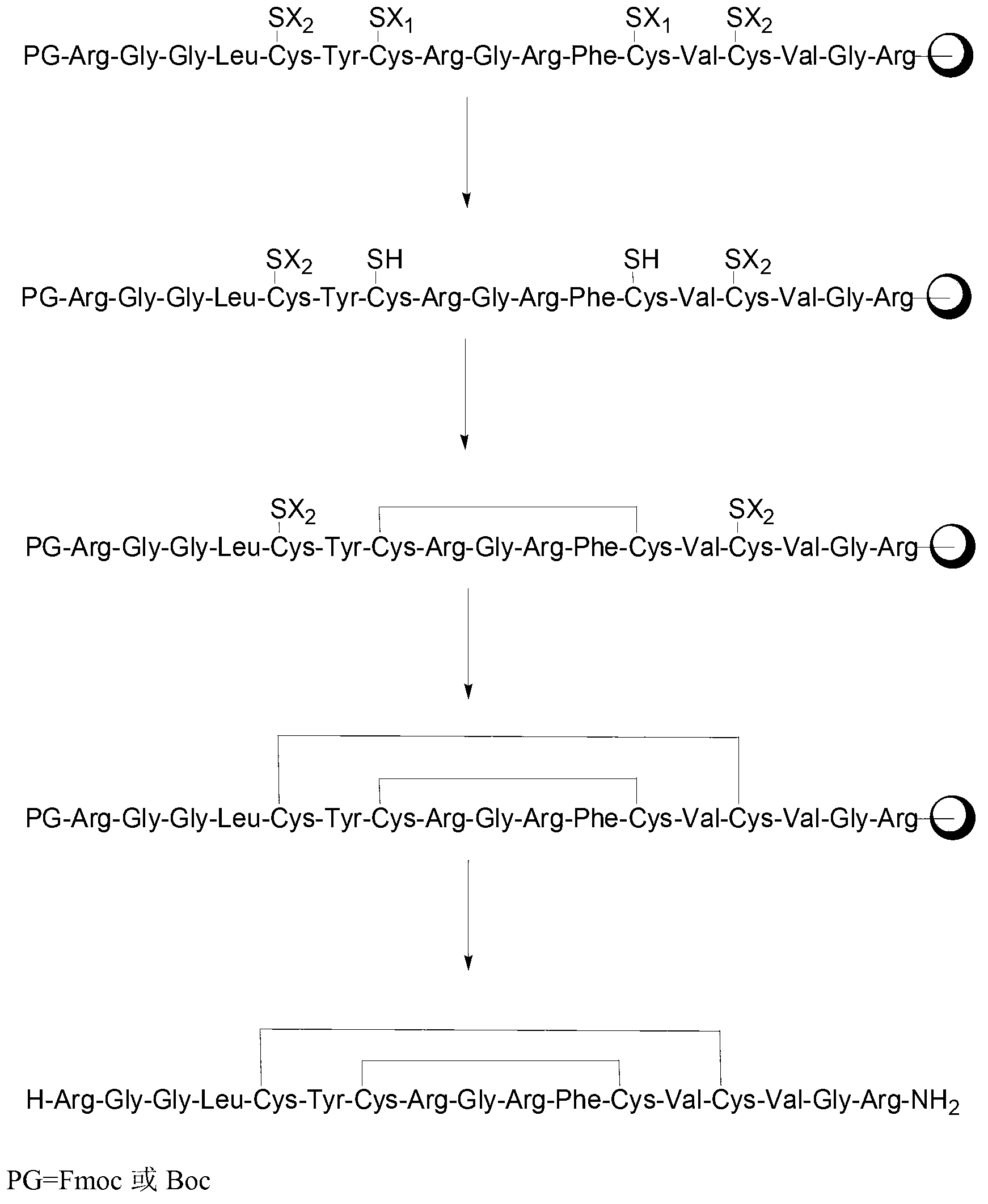
[0052] Fmoc-Arg(Pbf)-Gly-Gly-Leu-Cys(Acm)-Tyr(tBu)-Cys(Mmt)-Arg(Pbf)-Gly-Arg-Phe-Cys(Mmt)-Val-Cys(Acm) Preparation and Analysis of -Val-Gly-Arg(Pbf)-Amino Resin
[0053] (1) Preparation of H-Arg(Pbf)-amino resin
[0054] Weigh 2 g of Rink Amide resin with a substitution degree of 0.6 mmol / g in a solid phase reactor, add 10 mL of DCM to swell for 20 min, then add 10 mL of 20% piperidine in DMF, react for 5 min, wash with DMF once, add 10 mL of 20% piperidine DMF solution of pyridine, reacted for 15min. Drain. Add Fmoc-Arg(Pbf)-OH (3.6mmol), HOBt (4.3mmol), DIC (4.3mmol), DMF (15mL) into the solid phase reactor, and react at 20°C for 2h. Wash and dry the resin to obtain Fmoc-Arg(Pbf)-resin. Add 10 mL of blocking reagent (acetic anhydride (mmol): DIPEA (mmol): DMF = 1:1:8) to the resin, react for 1 h, block the remaining amino groups, wash with DCM, MeOH and DMF several times, and drain. Add 20% PIP / DMF solution and react for 20 minutes to remove the Fmoc protecting group, w...
Embodiment 2
[0065]
[0066] Weigh 3 g of the linear peptide resin obtained in step (2) of Example 1, add 30 mL of 1% TFA in DCM:DMSO=2:1 (volume ratio) solution to a solid phase reactor, and shake at 25°C for 4 hours. Drain. Wash with DMF and anhydrous methanol three times each, and drain. got Cys 5-14 A ring peptide resin:
[0067]
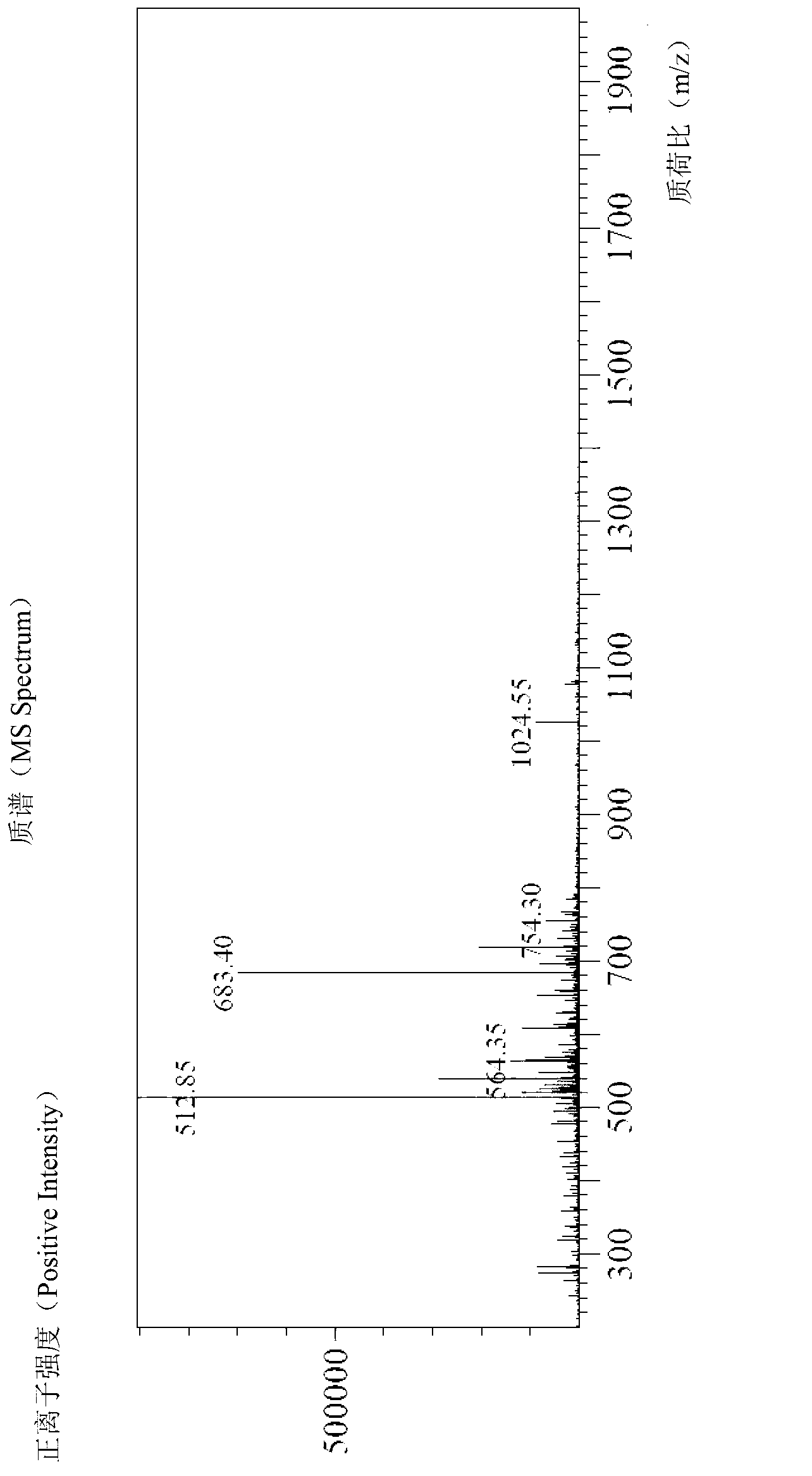
[0068] (2) Cys Glam 5-14 Analysis of a cyclic crude peptide
[0069] Weigh 0.5 g of the peptide resin obtained in step (1) of Example 2, add 5 mL of DCM to the solid phase reactor, swell for 10 min, and drain. Add 10 mL of 20% piperidine in DMF, react for 5 min, wash once with DMF, add 10 mL of 20% piperidine in DMF, and react for 15 min. Drain. They were washed twice with DMF and three times with anhydrous methanol, and drained. Prepare 5 mL of lysate, and the volume ratio of each component is TFA: sulfide anisole: TIS: anisole: water = 90: 2.5: 2.5: 2.5: 2.5. After shaking and reacting at 25° C. for 3 h, the reaction solution was poured into ...
Embodiment 3
[0070] The preparation and analysis of embodiment 3 Estherglam
[0071]
[0072]
[0073] Weigh 2 g of the peptide resin obtained in step (1) of Example 2, add 10 mL of DCM to the solid phase reactor, swell for 10 min, and drain. Add 10 mL of DMF. Dissolve 655.2 mg of iodine in 10 mL of MeOH and add it to the peptide resin. The mixture is shaken and reacted for 4 hours at room temperature, washed with DMF and anhydrous methanol three times each, and drained. got Cys 9-12 , Cys 5-14 Bicyclic peptide resin 1.78g.
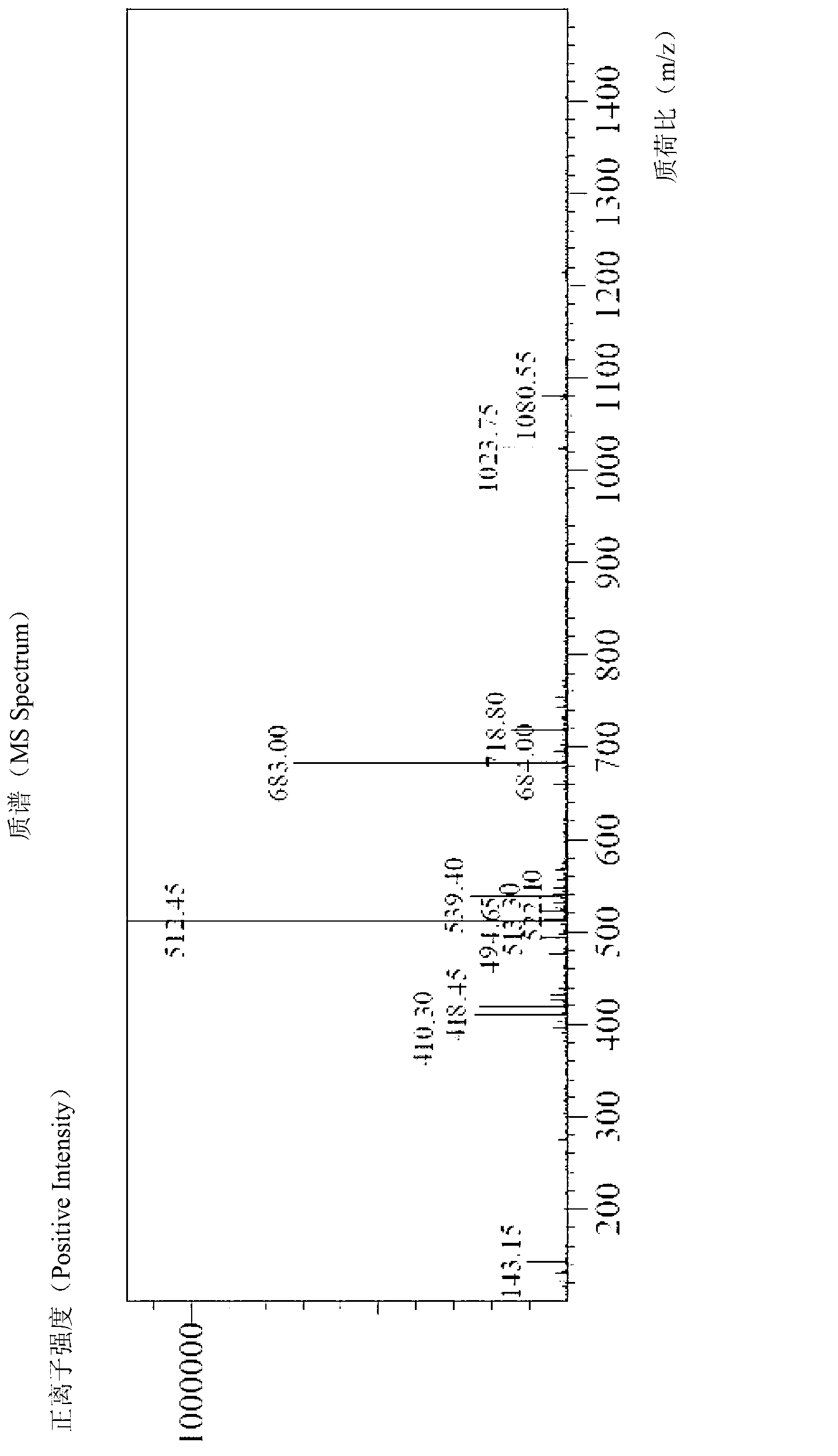
[0074] (2) Preparation of crude peptide of Esserglynan
[0075] Add 10 mL of 20% piperidine in DMF to the peptide resin obtained in step (1) of Example 3, react for 5 min, wash once with DMF, add 10 mL of 20% piperidine in DMF, and react for 15 min. Drain. They were washed twice with DMF and three times with anhydrous methanol, and drained. Prepare 20 mL of lysate, and the volume ratio of each component is TFA: sulfide anisole: TIS: anisole: water = 90: 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com