Battery grade sheet hydrated iron phosphate and preparation method thereof
A technology of hydrated ferric phosphate and hydrated phosphoric acid, which is applied in battery electrodes, circuits, electrical components, etc., can solve the problems of low rate performance and poor low temperature performance of lithium iron phosphate materials, and is suitable for large-scale mass production and low cost , The effect of reducing product cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Weigh 2g of cetyltrimethylammonium bromide, 13.90g of ferrous sulfate heptahydrate and 23.06g of concentrated phosphoric acid into 300ml of deionized water at room temperature, that is, the addition of surfactant is about 6.7g / L, iron The element concentration is 0.17mol / L, and the molar ratio of iron and phosphorus is 1:4.1. Then ultrasonically stir until completely dissolved. Then add 3.2g concentration of 28% hydrogen peroxide dropwise to the system. After the dropwise addition, continue to stir for 2 hours, and the pH value of the system is 1.2; The reaction system was filtered to obtain 289g of the filtrate, and the product was washed repeatedly with ethanol ultrasonics to obtain a powdery white precipitate; the powdery white precipitate was dried at 60°C for 8 hours to obtain 8.41g of the hydrated ferric phosphate of the present invention, with a yield of 90% .
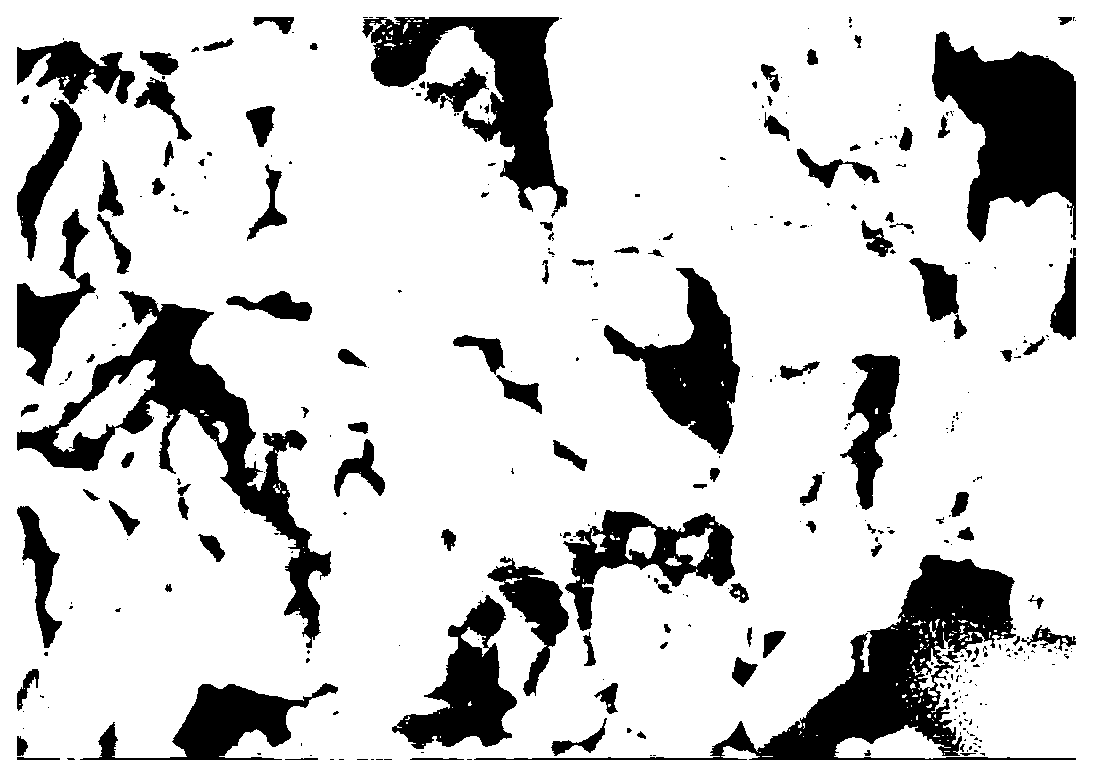
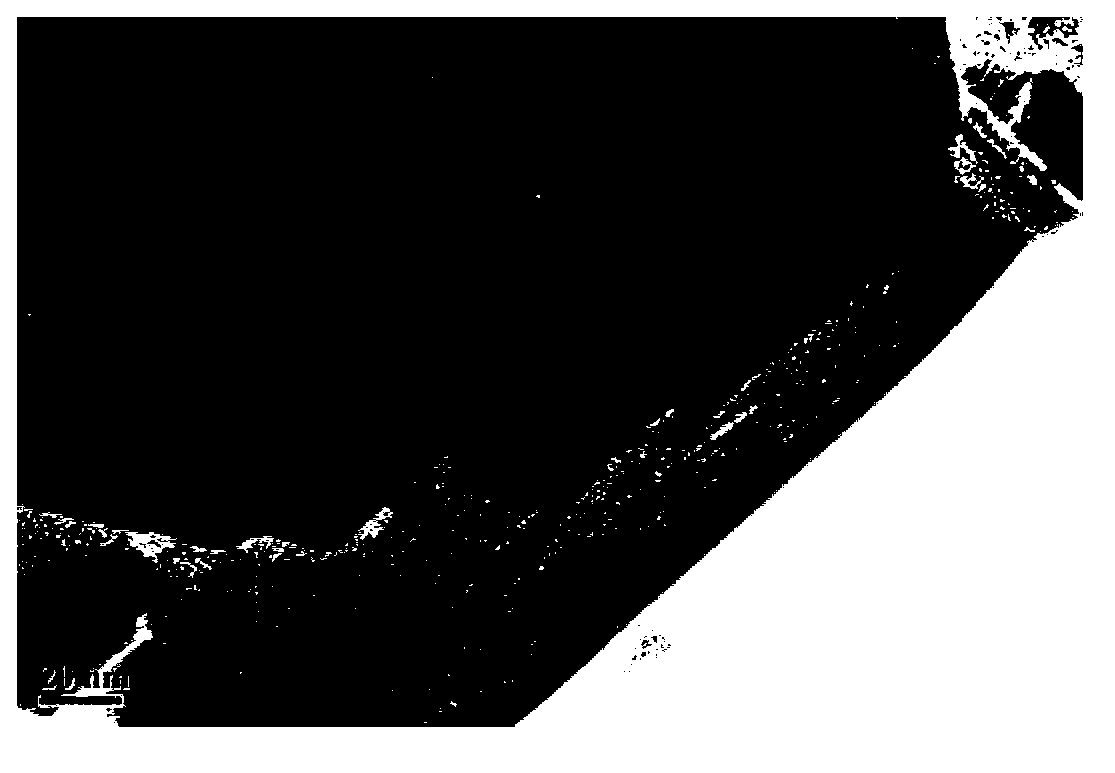
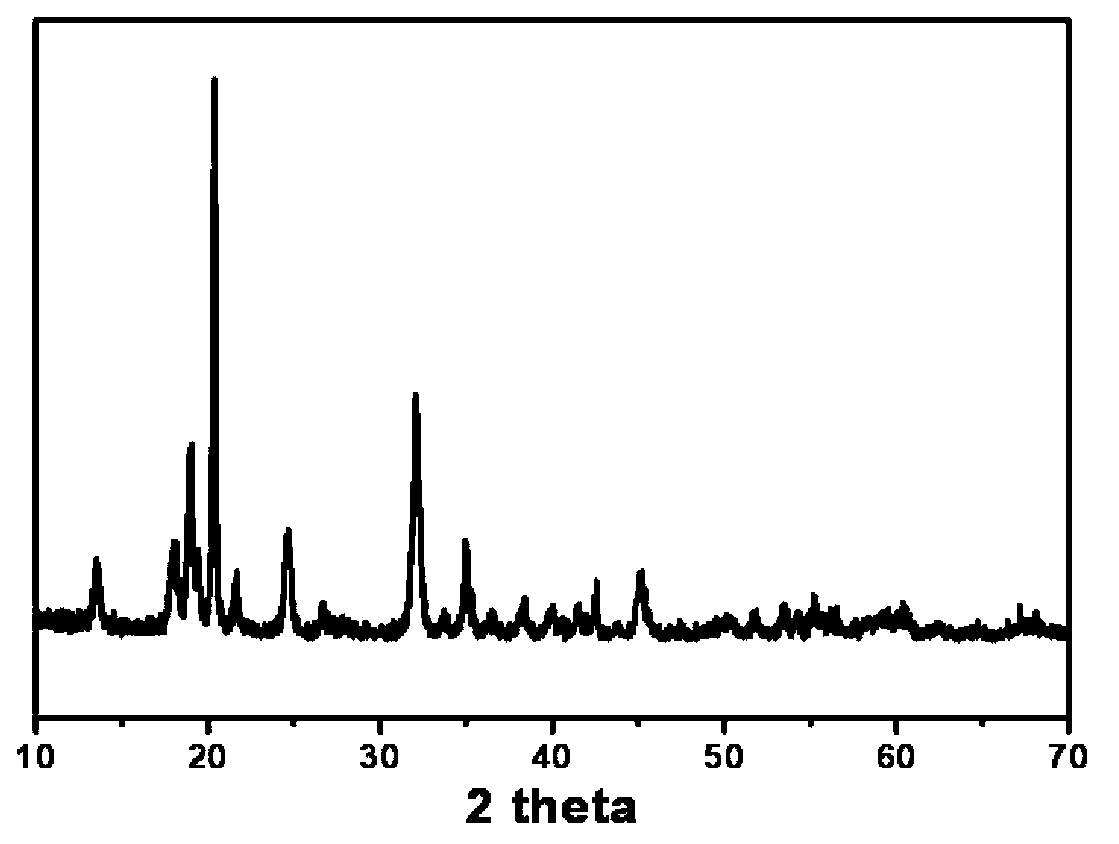
[0034] Scanning and transmission electron microscope observations are carried out to obtaining hydra...
Embodiment 2
[0036]Weigh 3g of cetyltrimethylammonium bromide, 5.46g of pure iron powder, 1.7g of ammonium dihydrogen phosphate and 31.2g of concentrated phosphoric acid into 300ml of deionized water at room temperature, that is, the amount of surfactant added is about 10g / L, the concentration of iron element is 0.32mol / L, and the molar ratio of iron element and phosphorus element is 1:5.0. Then ultrasonically stir until completely dissolved. Then add 3.3g of hydrogen peroxide with a concentration of 29% dropwise into the system. After the dropwise addition, continue to stir for 1 hour, and the pH value of the system is 1.8; then heat up to 80°C, and continue the reaction for 12 hours under stirring. The reaction system was filtered to obtain 291 g of filtrate, and the product was washed repeatedly with ethanol ultrasonics to obtain a powdery white precipitate; the powdery white precipitate was dried at 65° C. for 7 hours to obtain 8.27 g of the hydrated ferric phosphate of the present in...
Embodiment 3
[0038] Take by weighing 0.5g cetyltrimethylammonium bromide, 8.99g ferrous oxalate dihydrate, 5.65g concentrated phosphoric acid and 9g deionized water at room temperature and add them to the 291g filtrate in Example 2, namely the added surface active The amount of the agent is about 1.7g / L, the concentration of iron element is 0.27mol / L, and the molar ratio of iron element and phosphorus element is 1:1.01. Then ultrasonically stir until completely dissolved. Then add 3.1 g of hydrogen peroxide with a concentration of 30% dropwise to the system. After the dropwise addition, continue to stir for 1.5 hours, and the pH of the system is 1.75; then heat up to 90° C., and continue the reaction for 6 hours under stirring. The reaction system was filtered to obtain 285 g of the filtrate, and the product was washed repeatedly with ethanol ultrasonics to obtain a light pink precipitate; the light pink precipitate was dried at 70°C for 5 hours to obtain 8.67 g of the hydrated ferric phos...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com