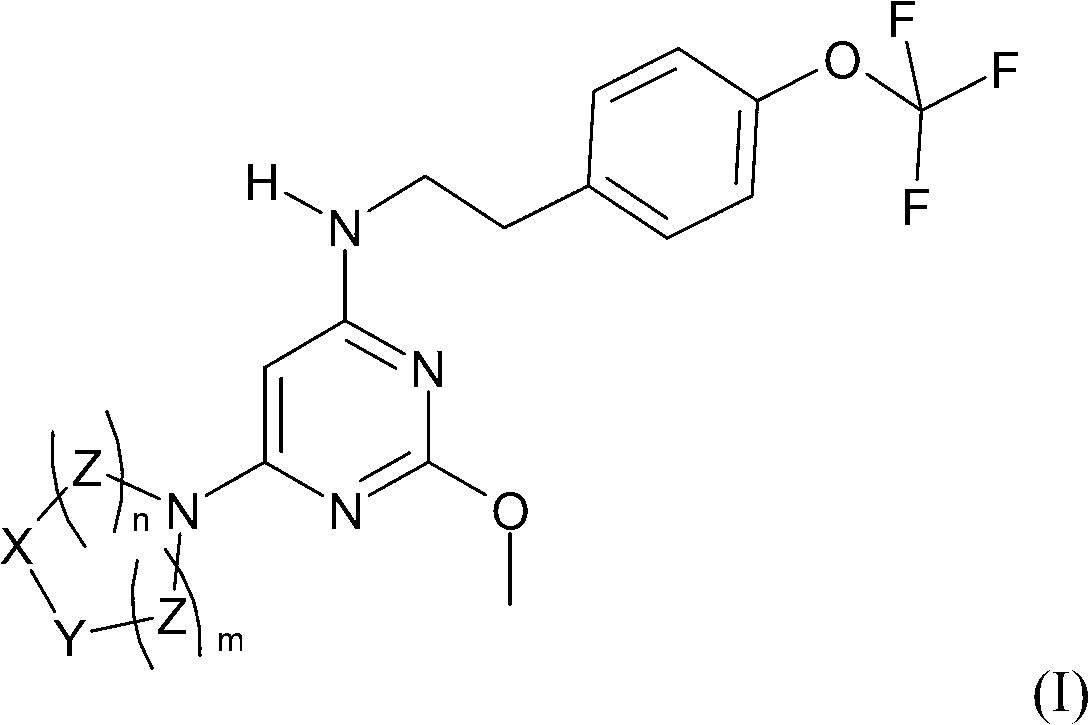
Substituted pyrimidines as prostaglandin D2 receptor antagonists
A compound and pyrimidine technology, applied in the field of substituted pyrimidine compounds, can solve the problems of discontinuation of niacin treatment, influence of patients' compliance with doctor's orders, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0136] 4-{2-Methoxy-6-[2-(4-trifluoromethoxy-phenyl)-ethylamino]-pyrimidin-4-yl}-morpholine-2-carboxylic acid trifluoroacetate
[0137]
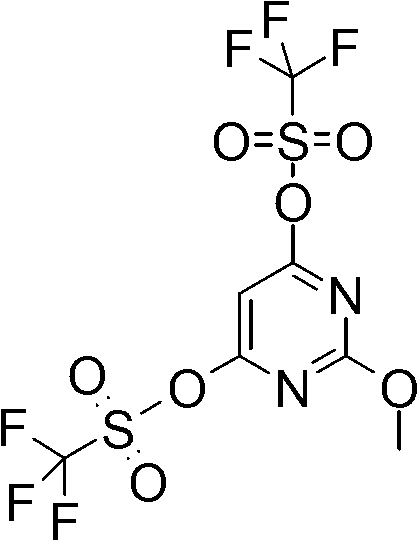
[0138] 1A. 2-Methoxy-6-[2-(4-trifluoromethoxy-phenyl)-ethylamino]-pyrimidin-4-yl trifluoromethanesulfonate
[0139]
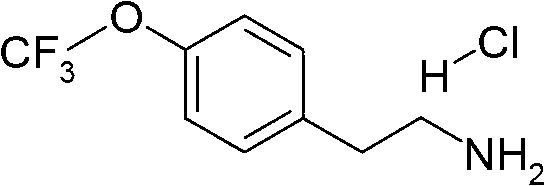
[0140] At room temperature, mix 2-methoxy-6-trifluoromethanesulfonyl-pyrimidin-4-yl trifluoromethanesulfonate (105 mg, 0.26 mol) and 2-(4-trifluoromethoxy-phenyl) -CH of ethylamine (53mg, 0.26mmol) 2 Cl 2 (3 mL) and the solution was stirred overnight. More 2-(4-trifluoromethoxy-phenyl)-ethylamine (ca. 100 mg) was added. After 2 hours, LC / MS indicated that the reaction was complete. The reaction mixture was concentrated in vacuo and the crude product was used in the next step without further purification. LC Rt: 1.19 min; MS 462 (M+1).
[0141] 1B. 4-{2-methoxy-6-[2-(4-trifluoromethoxy-phenyl)-ethylamino]-pyrimidin-4-yl}-morpholine-2-carboxylic acid ethyl ester
[0142]
[0143] At 95°C, 2-methoxy-6-[2-(4-trifl...
Embodiment 2
[0149] (4-{2-Methoxy-6-[2-(4-trifluoromethoxy-phenyl)-ethylamino]-pyrimidin-4-yl}-piperazin-1-yl)-acetic acid tri Trifluoroacetate
[0150]
[0151] 2A. [4-(6-{tert-butoxycarbonyl-[2-(4-trifluoromethoxy-phenyl)-ethyl]-amino}-2-methoxy-pyrimidin-4-yl)- Piperazin-1-yl]-ethyl acetate
[0152]
[0153] At 40°C, trifluoromethanesulfonic acid 6-{tert-butoxycarbonyl-[2-(4-trifluoromethoxy-phenyl)-ethyl]-amino}-2-methoxy-pyrimidine-4 CH 2 Cl 2 (5 mL) and the solution was heated overnight. The reaction mixture was concentrated in vacuo and the residue was purified on silica gel with MeOH / CH 2 Cl 2 (2-3%) eluted to give the title product (37 mg, 36%). LC Rt: 3.59 min; MS 584 (M+1).
[0154] 2B.(4-{2-Methoxy-6-[2-(4-trifluoromethoxy-phenyl)-ethylamino]-pyrimidin-4-yl}-piperazin-1-yl)- Ethyl acetate hydrochloride
[0155]
[0156] At room temperature, [4-(6-{tert-butoxycarbonyl-[2-(4-trifluoromethoxy-phenyl)-ethyl]-amino}-2-methoxy-pyrimidin-4-yl )-piperazin-1-yl]-ethyl ...
Embodiment 3
[0162] 1-{2-Methoxy-6-[2-(4-trifluoromethoxy-phenyl)-ethylamino]-pyrimidin-4-yl}-piperidine-2-carboxylic acid trifluoroacetate
[0163]
[0164] 3A.1-(6-{tert-butoxycarbonyl-[2-(4-trifluoromethoxy-phenyl)-ethyl]-amino}-2-methoxy-pyrimidin-4-yl)-piper Ethyl pyridine-2-carboxylate
[0165]
[0166] At 85 ° C, trifluoromethanesulfonic acid 6-{tert-butoxycarbonyl-[2-(4-trifluoromethoxy-phenyl)-ethyl]-amino}-2-methoxy-pyrimidine-4 A solution of the -yl ester (144 mg, 0.26 mmol) and ethyl piperidine-2-carboxylate hydrochloride (99 mg, 0.51 mmol) and DIEA (89 μL, 0.51 mmol) in DMF (3 mL) was heated overnight. The reaction mixture was concentrated in vacuo and the residue was dissolved in CH 2 Cl 2 and water. The two layers were separated, and the organic layer was washed with 10% citric acid, saturated NaHCO 3 , water and saturated brine, washed with Na 2 SO 4 Dry, filter and concentrate in vacuo. The crude product was purified on silica gel eluting with EtOAc / heptane (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com