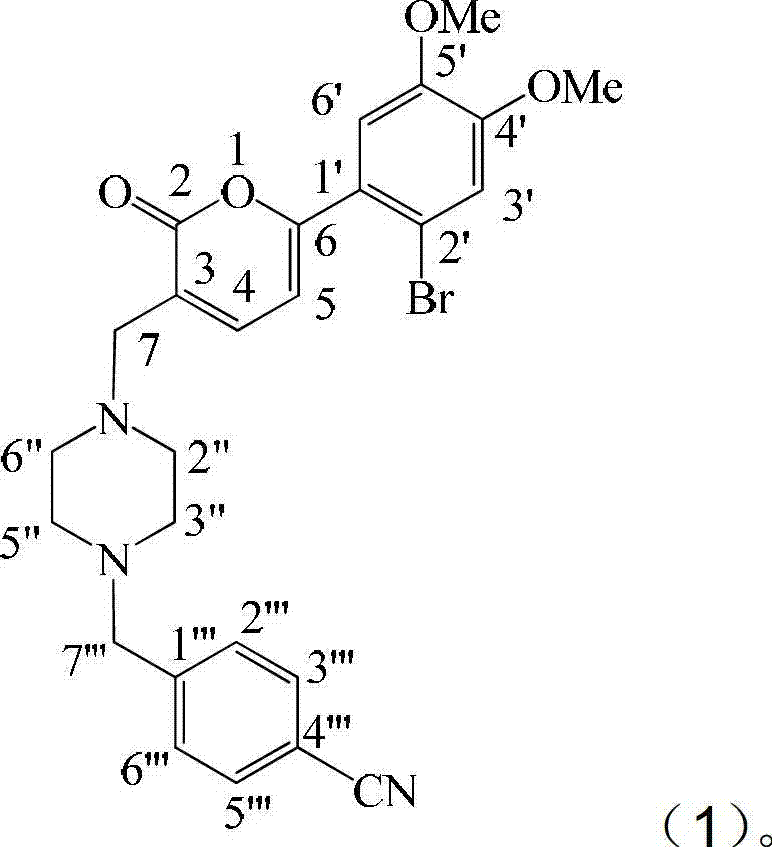
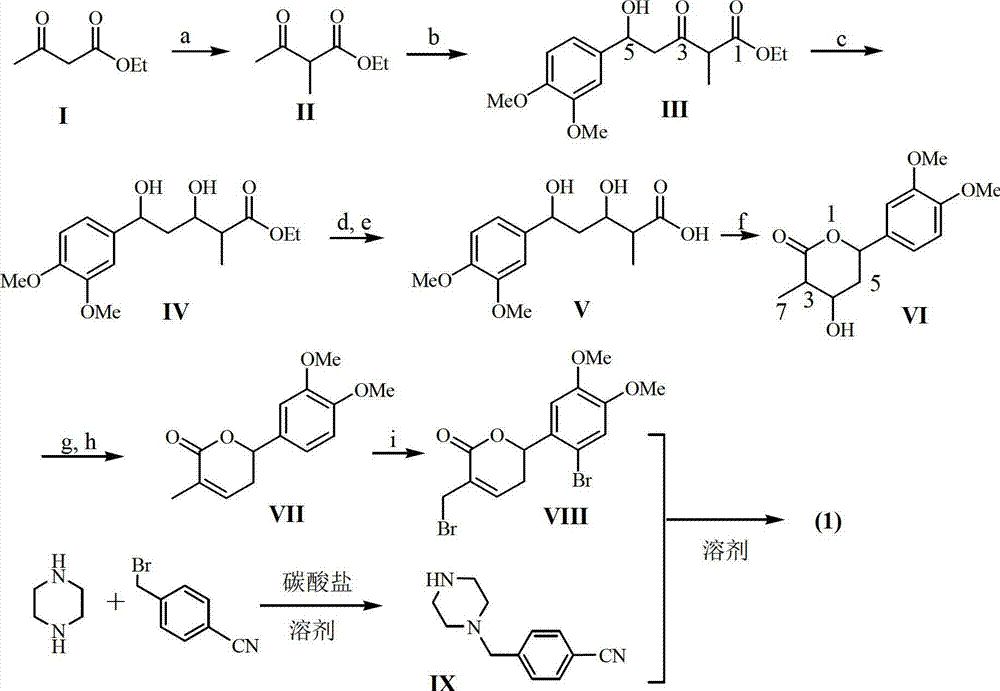
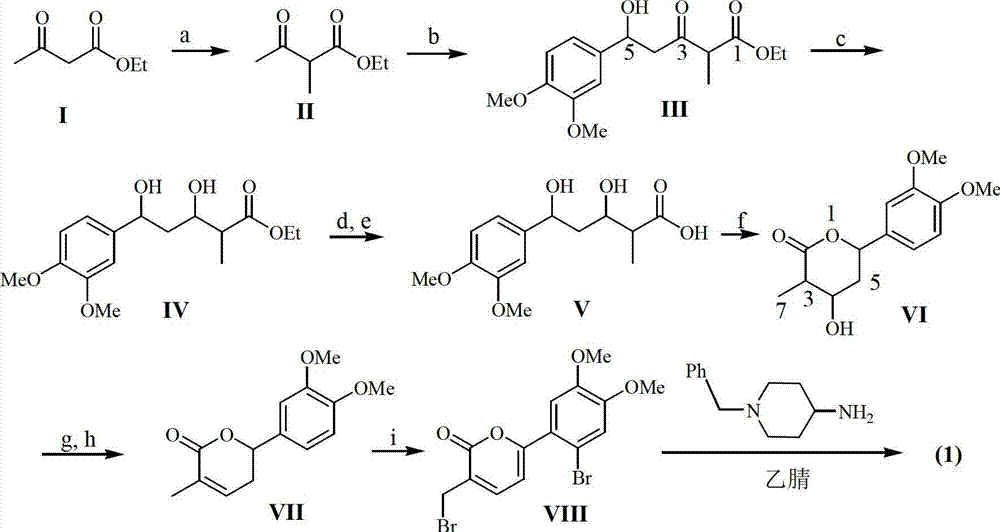
Application of substituted cyanobenzene in preparation of fungal infection resisting drugs
An antifungal and drug technology, applied in the field of medicine, can solve the problems of poor bioavailability, low water solubility, inconvenient use for patients, etc., achieve huge social and economic benefits, and inhibit the growth of fungi.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: Preparation of formula (1) compound
[0019] 1.1 Instruments and reagents
[0020] The melting point was measured with a microscopic melting point apparatus (produced by Beijing Tektronix Co., Ltd.), and the temperature was not corrected; the optical rotation was measured on a Polax-2L automatic polarimeter made in Japan; the infrared spectrum IR was measured by a Bruker Vector-22 infrared spectrometer, and pressed by KBr; Spectra were measured with a Shimadzu UV-240 ultraviolet spectrophotometer; hydrogen nuclear magnetic resonance 1 H NMR, carbon nuclear magnetic resonance 13 C NMR and 2D NMR were measured by INOVA superconducting nuclear magnetic resonance spectrometer (VARIAN INOVA-400MHz) (tetramethylsilyl ether TMS as internal standard); electrospray mass spectrometry ESI-MS was measured by Bruker Esquire 3000+ mass spectrometer, column layer Silica gel for analysis (100-200, 200-300 and 300-400 mesh) and silica gel GF254 for thin layer chromatograph...
Embodiment 2
[0044] Example 2: Antifungal activity test of compound of formula (1)
[0045] With reference to the standardized antifungal susceptibility test method proposed by the National Committee for Clinical Laboratory Standards (NCCLS), the compound 3-[4-(4-cyanobenzyl)-piperazine- In vitro antifungal activity of 1-yl]-methyl-6-(2-bromo-4,5-dimethoxyphenyl)-2H-pyran-2-one.
[0046] 2.1 Fungal standard strain:
[0047] Candida albicans ATCC10231: Provided by Chongqing Center for Disease Control;
[0048] Candida albicans ATCC76615: Provided by Changzheng Hospital Affiliated to Second Military Medical University.
[0049] 2.2 Reagents:
[0050] 2.2.1 Sabouraud dextrose agar medium (sabouraud dextrose agar): product of Guangdong Kai Microbiology Technology Co., Ltd.
[0051] 2.2.2 Yeast extract: repackaged by Haishenggong Bioengineering Technology Service Co., Ltd.
[0052] 2.2.3 Three azamorphine propanesulfonic acid (3-N-morpholinopropanesulfonic acid, MOPS).
[0053] 2.2.4 Te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com