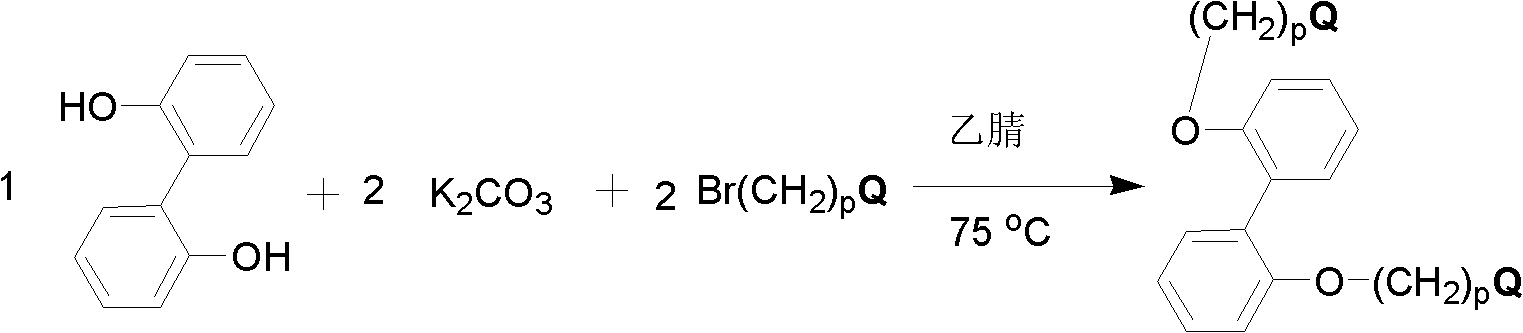Qaternized aromatic compound, polyketone with quaternary aromatic groups and methods for preparing quaternized aromatic compound and polyketone with quaternary aromatic groups
A technology of aromatic compounds and aromatic groups, which is applied in the field of polymers and their preparation, can solve problems such as complicated steps and difficulty in introducing quaternary ammonium groups, and achieve the effects of low cost, good mechanical properties and temperature resistance, and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] Embodiment 1: the synthesis of quaternized diaryl monomer
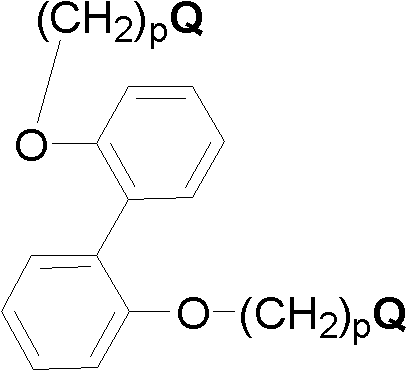
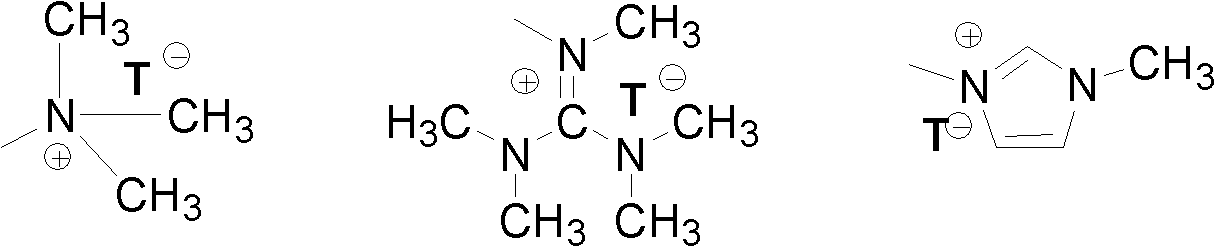
[0111] Add 1.0 moles of 2,2'-dihydroxybiphenyl, 2.0 moles of potassium carbonate, 2.0 moles of 4-bromobutyltrimethylammonium bromide and 15 liters of acetonitrile into the 25 liter reaction flask, mechanically stirred at 75°C for 24 hours, filtered, and the solvent was removed by distillation under reduced pressure. Oxydiphenyl dibromide, abbreviated as DNODP, 1 H NMR (400MHz, D 2 O) δ (ppm): 7.41 (Ar-H4, t, 2H), 7.27 (Ar-H6, d, 2H), 7.09-7.16 (Ar-H3 & Ar-H5, m, 4H), 4.03 (OCH 2 , t, 4H), 3.06 (CH 2 N, t, 4H), 2.88 (NCH 3 , t, 18H), 1.52-1.70 (CH 2 CH 2 CH 2 CH 2 , m, 8H), confirmed the structure of compound, reaction formula is as follows:
[0112]
[0113] Other quaternized diaryl monomers containing different alkyl lengths and quaternary ammonium groups were synthesized in a similar manner, except that different quaternizing agents were used instead of 4-bromobutyltrimethylammonium bromide.
Embodiment 2
[0115]0.01 mole of DNODP in Example 1, 1.00 mole of 4,4'-diphenyl ether dicarboxylic acid, 0.99 mole of 2,2'-dimethoxybiphenyl and 2 liters of trifluoromethanesulfonic acid were added to the In a nitrogen reaction flask, stir and react at 40°C for 24 hours, cool the reaction solution and pour it into an ice / water mixture to precipitate a polymer, filter and wash repeatedly with deionized water until the filtrate is neutral, and use After soaking in 1mol / L sodium hydroxide aqueous solution at room temperature for 24 hours, then vacuum-dry at room temperature to obtain OH - Polymers with side chains containing quaternary ammonium groups in the form of polymers. A strong carbonyl absorption peak appears in the infrared spectrogram and almost no carboxyl absorption peak is observed, indicating the occurrence of polyacylation; the proton nuclear magnetic resonance spectrum confirms the structure of the obtained polymer and the ratio of each structural unit calculated by the integra...
Embodiment 3
[0117] 0.50 moles of DNODP in Example 1, 1.00 moles of 4,4`-diphenyl ether dicarboxylic acid, 0.50 moles of 2,2`-dimethoxybiphenyl and 2 liters of trifluoromethanesulfonic acid were added to the In a nitrogen reaction flask, stir and react at 60°C for 24 hours. After cooling the reaction solution, pour it into an ice / water mixture to precipitate a polymer. Filter and wash repeatedly with deionized water until the filtrate is neutral. After soaking in 1mol / L sodium hydroxide aqueous solution at room temperature for 24 hours, then vacuum-dry at room temperature to obtain OH - Polymers with side chains containing quaternary ammonium groups in the form of polymers. A strong carbonyl absorption peak appears in the infrared spectrogram and almost no carboxyl absorption peak is observed, indicating the occurrence of polyacylation; the proton nuclear magnetic resonance spectrum confirms the structure of the obtained polymer and the ratio of each structural unit calculated by the integ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Intrinsic viscosity | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com