Amphiphilic conjugate nano-particle for treating tumors, as well as preparation method and application of same
A nanoparticle and amphiphilic technology, applied in the field of biomedicine, can solve problems such as difficult control and uneven size of drug carriers, and achieve the effect of reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
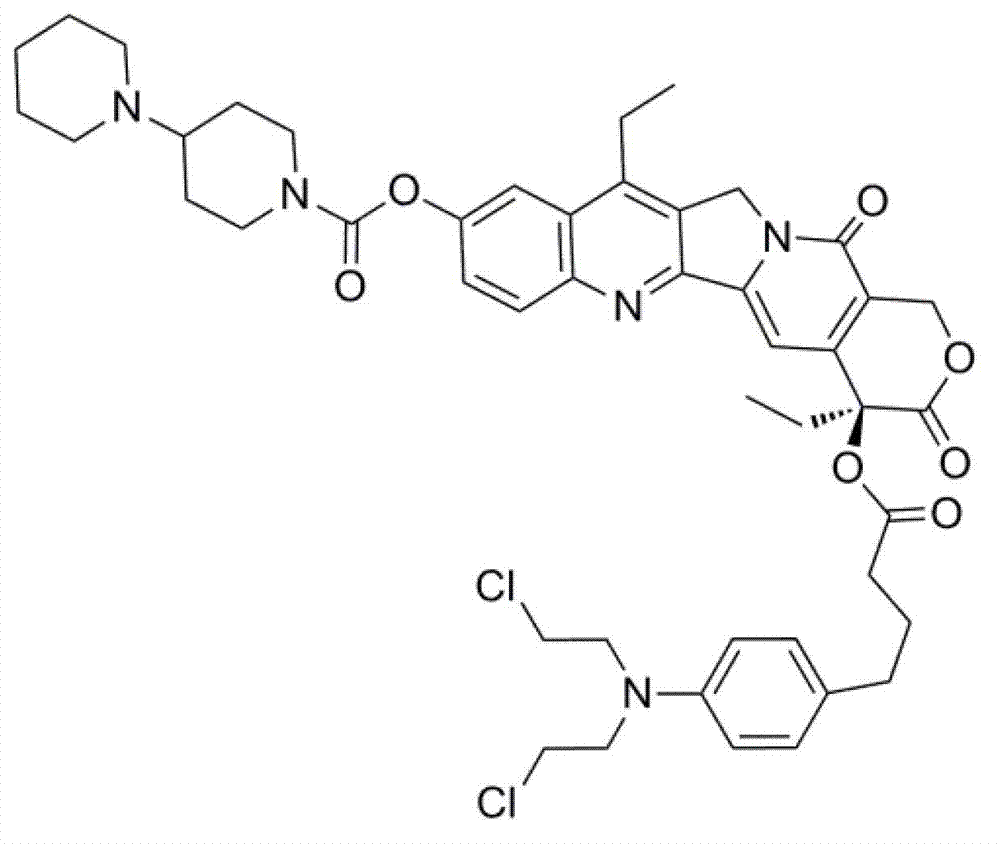
[0035] Chlorambucil (408 mg), irinotecan (158 mg), N,N′-dicyclohexylcarbodiimide (DCC, 330 mg) and 4-dimethylaminopyridine (DMAP, 74 mg) Add it into the reaction bottle, then add 20 ml of chloroform, and stir at room temperature for 48 hours. Remove the precipitated insoluble matter dicyclohexyl urea (DCU) by filtration, use a mixture of dichloromethane and methanol (20:1) as eluent in the filtrate, and separate it by column chromatography to obtain phenylbutyrate nitrogen in the form of light yellow powder Mustard-irinotecan conjugates, yield 67%.
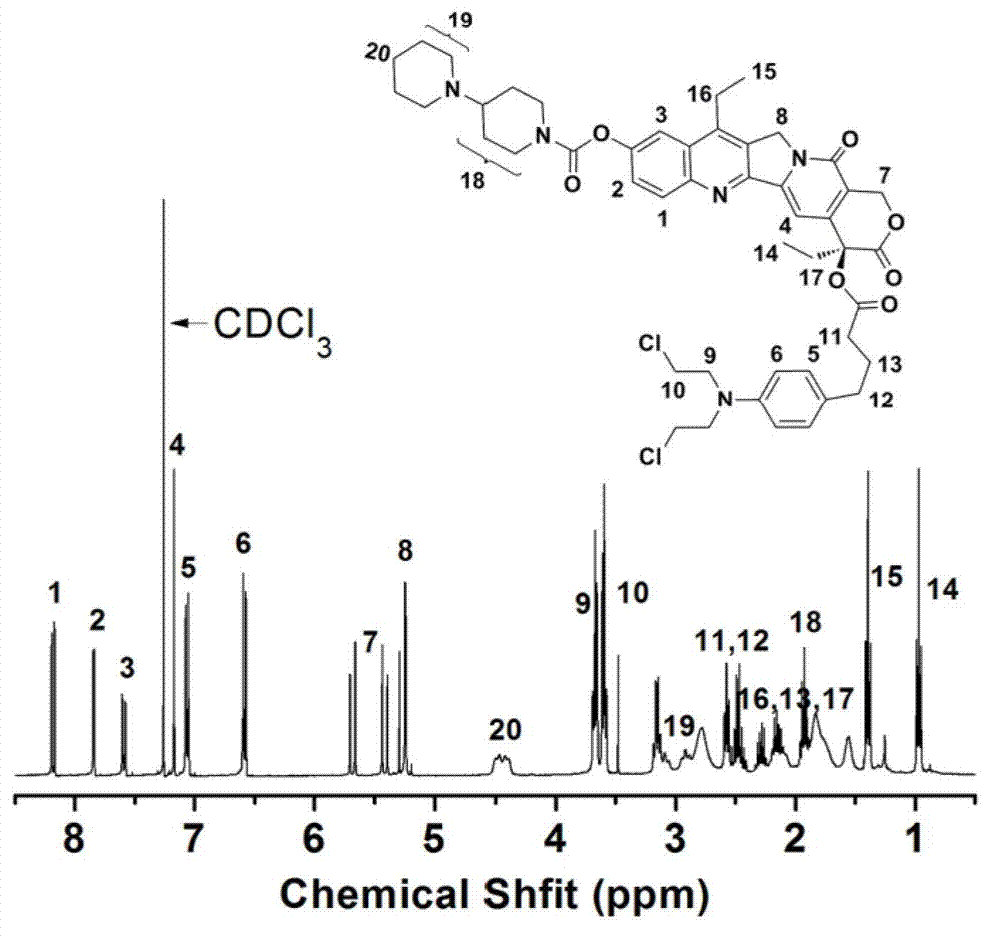
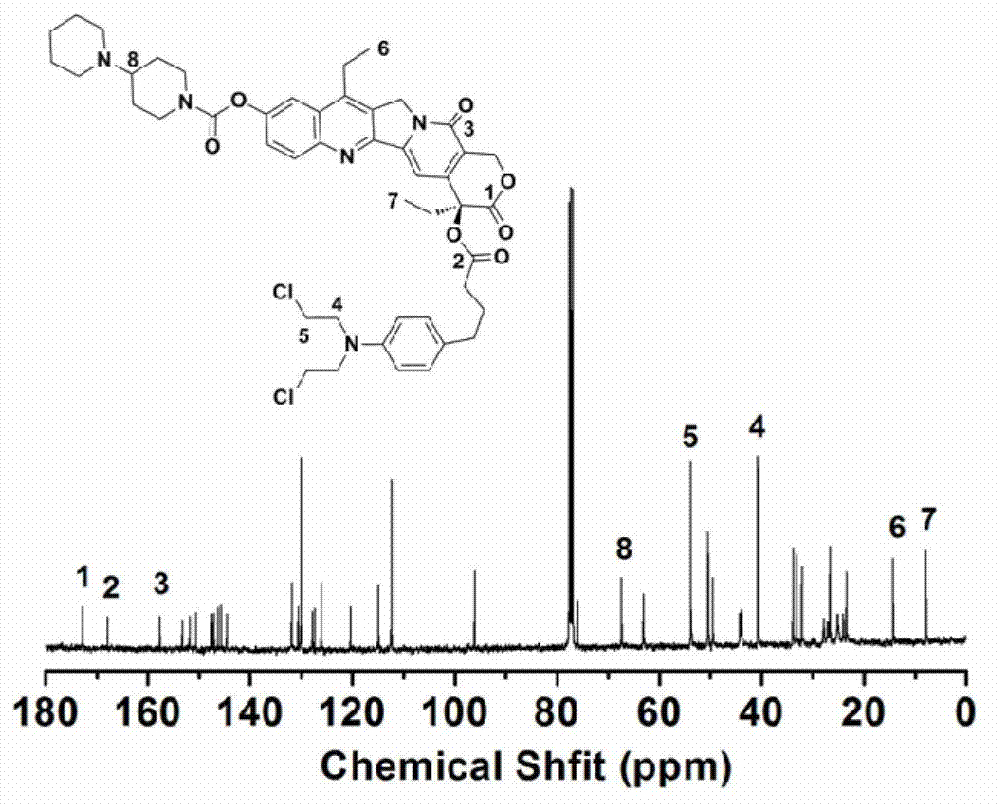
[0036] The chemical structure of the conjugate synthesized in this example is as follows: figure 1 shown. of the conjugates prepared in this example 1 H NMR and 13 C NMR spectrum as figure 2 and image 3 As shown, the test solvent is CDCl 3 , each absorption peak was assigned and marked in the spectrum. spectrum figure 2 The assignment of the proton peaks in is as follows: δ (ppm from TMS), 8.17-8.19 (1H, CHCHCCCHC), 7....
Embodiment 2
[0039] Chlorambucil (304 mg) was dissolved in 3 mL of thionyl chloride and reacted at 0°C for 3 hours. The thionyl chloride was removed by distillation under reduced pressure to give the chlorambucil chloride product (tan oil). Then the acid chloride product was dissolved in 5 ml of chloroform, slowly added dropwise to a solution of topotecan (630 mg) and triethylamine (144 μl) in 20 ml of chloroform, and reacted at room temperature for 24 hours. The precipitated white triethylamine hydrochloride was removed by filtration, and the filtrate used a mixture of dichloromethane and methanol (15:1) as the eluent, and was separated by column chromatography to obtain a light yellow powder chlorambucil-topology Tecan conjugate, yield 49%.
[0040] The chemical structure of the drug / drug conjugate synthesized in this example is as follows: Figure 5 shown.
[0041] The amphiphilic conjugate prepared above was dissolved in tetrahydrofuran, which was dropped into water at room temperat...
Embodiment 3
[0043] Add pintamustine (358 mg), irinotecan (158 mg), N,N′-dicyclohexylcarbodiimide (DCC, 309 mg) and 4-dimethylaminopyridine (DMAP, 74 mg) The reaction bottle was added with 20 ml of chloroform, and the reaction was stirred at room temperature for 48 hours. The precipitated insoluble dicyclohexyl urea (DCU) was removed by filtration, and the filtrate used a mixture of dichloromethane and methanol at a volume ratio of (20:1) as the eluent, and was separated by column chromatography to obtain a light yellow powder of Binda nitrogen mustard - Irinotecan conjugate, yield 64%.
[0044] The chemical structure of the conjugate synthesized in this example is as follows: Figure 6 shown.
[0045] The amphiphilic conjugate prepared above was dissolved in tetrahydrofuran, which was dropped into water at room temperature, and the tetrahydrofuran was removed to obtain an aqueous solution of nanoparticles of the amphiphilic conjugate. The average size of the anti-tumor nanoparticles pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com