Hybridoma cell strain and monoclonal antibody of anti-heart-type fatty acid-binding protein generated by same
A hybridoma cell line, monoclonal antibody technology, applied in the direction of anti-animal/human immunoglobulin, microorganisms, biological testing, etc., can solve the problems of high price and difficult experimentation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
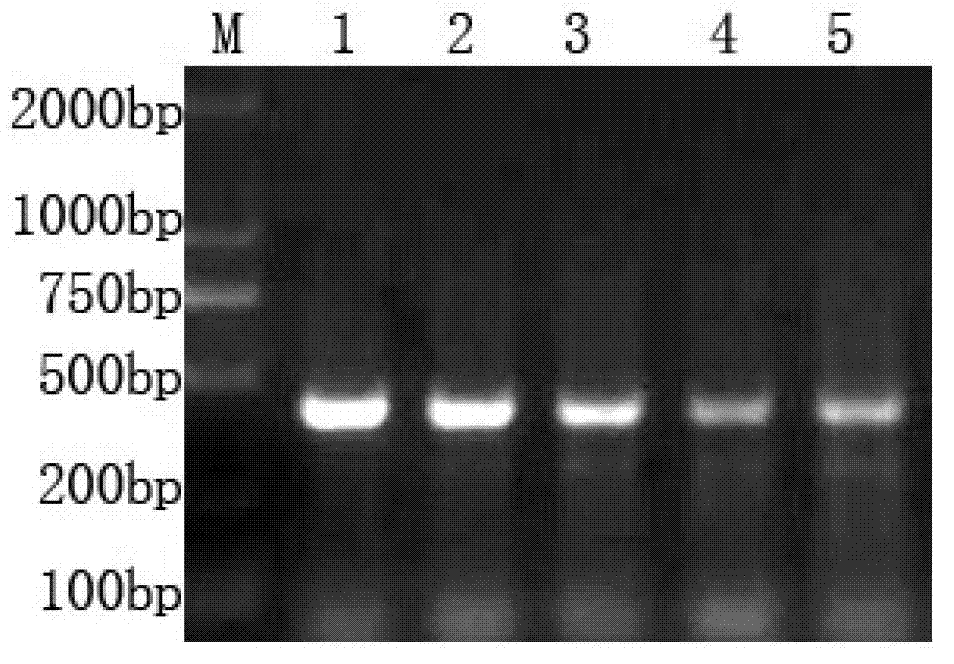
[0040] The preparation of embodiment 1.H-FABP recombinant protein
[0041] 1) Obtain the whole gene of H-FABP coding sequence
[0042] According to the H-FABP gene sequence (NM_004102) provided by GeneBank, use the software to compare with the liver type, small intestine type, brain type, silver snow disease-related type and adipocyte type fatty acid binding protein gene sequence, observe the homology, and determine the heart type The pseudo-cloned sequence of the fatty acid binding protein gene (SEQ ID NO: 1):
[0043] ATGGTGGACG CTTTCCTGGG CACCTGGAAG CTAGTGGACA GCAAGAATTT CGATGACTAC 60
[0044] ATGAAGTCAC TCGGTGTGGG TTTTGCTACC AGGCAGGTGG CCAGCATGAC CAAGCCTACC 120
[0045] ACAATCATCG AAAAGAATGG GGACATTCTC ACCCTAAAAA CACACAGCAC CTTCAAGAAC 180
[0046] ACAGAGATCA GCTTTAAGTT GGGGGTGGAG TTCGATGAGA CAACAGCAGA TGACAGGAAG 240
[0047] GTCAAGTCCA TTGTGACACT GGATGGAGGG AAACTTGTTC ACCTGCAGAA ATGGGACGGG 300
[0048] CAAGAGACCA CACTTGTGCG GGAGCTAATT GATGGAAAAC TCATCCTGAC ACTCACCCAC ...
Embodiment 2
[0076] Example 2. Bioinformatics analysis and determination of detection targets
[0077] 1) Find the amino acid sequence of H-FABP protein from GENEBANK, which consists of 133 amino acid residues.
[0078] 2) Analyze the potential B cell epitope region in the H-FABP protein online, comprehensively consider the secondary structure, antigenicity, hydrophilicity, accessibility and flexibility of the protein, analyze and score each segment, and select the one with the highest score A region is used as a candidate B cell epitope region, which is 32aa-47aa (peptide 1, the amino acid sequence is represented by SEQ ID NO: 2: QVASMTKPTTIIEKNG)
Embodiment 3
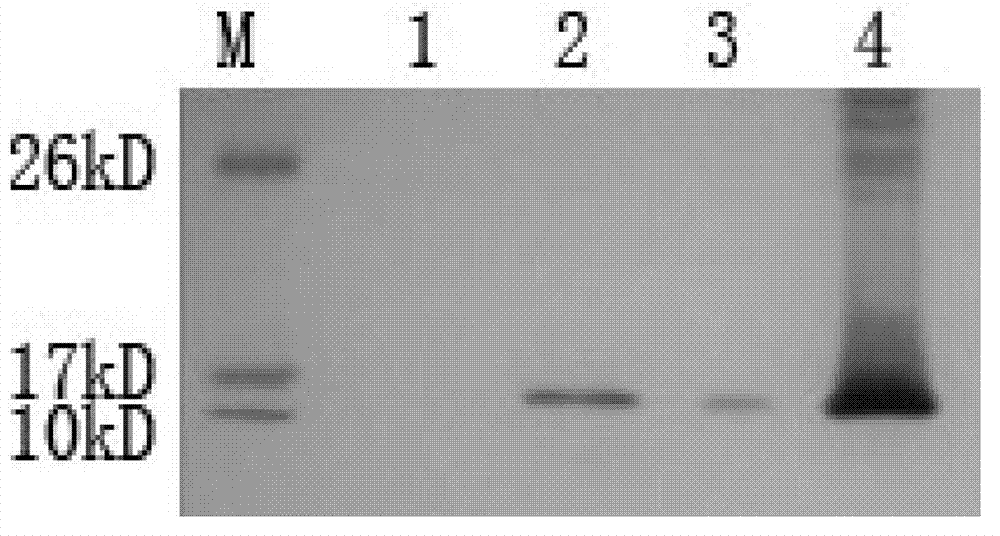
[0079] Example 3. Preparation of anti-H-FABP monoclonal antibody
[0080] 1) Synthesis and cross-linking of epitope peptides
[0081] H-FABP B-cell epitope peptide 1 was chemically synthesized, purified and cross-linked with BSA to obtain H-FABP-peptide 1-BSA
[0082] 2) Preparation of H-FABP monoclonal antibody
[0083] The cross-linked product of the synthesized H-FABP epitope peptide and BSA and the recombinant protein H-FABP were emulsified with an equal amount of Freund's complete adjuvant, and the antigen emulsification was carried out by double syringes. After the emulsification was completed, 5 Balb / c mice aged about 8 weeks were given basic immunization (100 μg / ml) by multi-point subcutaneous injection in limbs and intraperitoneal injection. Two weeks later, emulsify the H-FABP whole antigen with the same amount of Freund's incomplete adjuvant, and immunize 5 Balb / c mice by multi-point subcutaneous injection in limbs and intraperitoneal injection (100 μg / ml); Two w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Affinity | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com