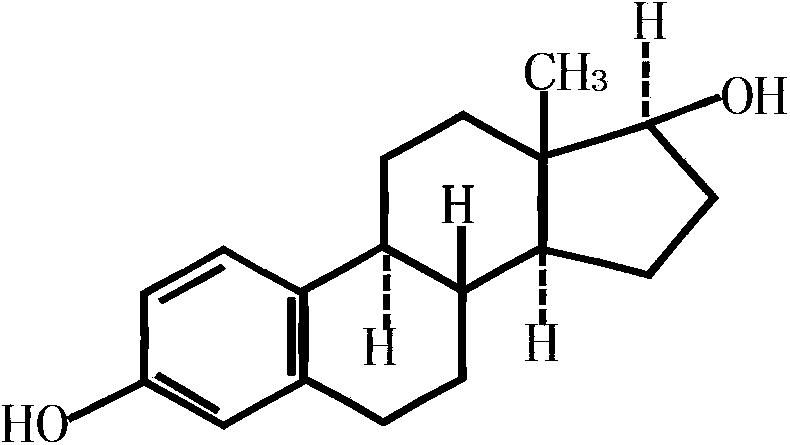
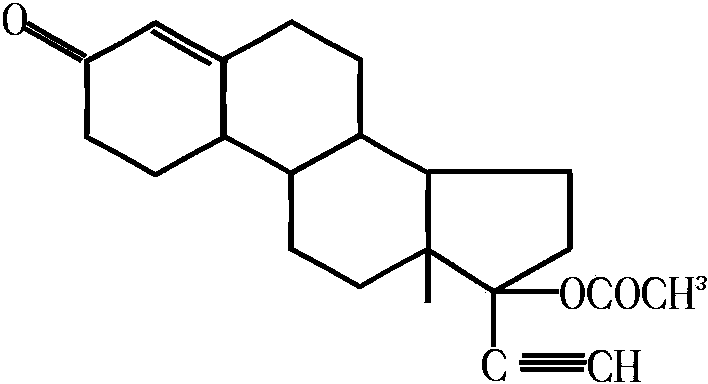
Norethisterone acetate-containing compound estradiol transdermal sustained release preparation and preparation method thereof
A technology of norethisterone acetate and compound estradiol, which is applied in the field of medicine, can solve problems such as embarrassment in the promotion and use of estrogen, stimulation of endometrial hyperplasia, etc., and achieves avoiding the phenomenon of peak and valley of blood drug concentration, decrease of blood drug concentration, The effect of reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A kind of compound estradiol transdermal slow-release preparation containing norethindrone acetate of the present embodiment comprises a backing layer, a protective film and a skeleton layer between the backing layer and the protective film; the skeleton layer includes an upper layer A skeleton layer, a lower skeleton layer and a controlled-release membrane between the upper skeleton layer and the lower skeleton layer; the surface of the lower skeleton layer is covered with a backing layer made of aluminum-plastic film.
[0037] Its preparation method is: 1) 1g estradiol, 10.5g norethindrone acetate, 25.46g laurocapram, 100.97g propylene glycol, 14.05g lauric acid, 18.6g polyethylene glycol 400, 287.86g polyacrylate and Mix 46.97g of ethyl acetate, stir evenly, and place until the bubbles disappear; 2) Apply the mixture obtained in step 1) on the anti-adhesive layer with a thickness of 0.10mm, and place it in an oven to dry at a temperature of 80°C, drying time is 60 mi...
Embodiment 2
[0041] A kind of compound estradiol transdermal slow-release preparation containing norethindrone acetate of the present embodiment, a kind of compound estradiol transdermal slow-release preparation containing norethindrone acetate of the present embodiment, comprises backing layer, protection film and a skeleton layer between the backing layer and the protective film; the skeleton layer includes an upper skeleton layer, a lower skeleton layer and a controlled release membrane between the upper skeleton layer and the lower skeleton layer; the lower skeleton layer The surface is covered with a backing layer made of aluminum-plastic film.
[0042] Its preparation method is: 1) 1.5g estradiol, 12g norethindrone acetate, 24.16g laurocaprone, 79.68g propylene glycol, 10.69g lauric acid, 20.67g polyethylene glycol 400, 268.91g polyacrylate and Mix 53.98g of ethyl acetate, stir evenly, and place until the air bubbles disappear; 2) Apply the mixture obtained in step 1) on the anti-adh...
Embodiment 3
[0046] A kind of compound estradiol transdermal slow-release preparation containing norethindrone acetate of the present embodiment comprises a backing layer, a protective film and a skeleton layer between the backing layer and the protective film; the skeleton layer includes an upper layer A skeleton layer, a lower skeleton layer and a controlled-release membrane between the upper skeleton layer and the lower skeleton layer; the surface of the lower skeleton layer is covered with a backing layer made of aluminum-plastic film.
[0047] Its preparation method is: 1) 2g estradiol, 13.5g norethindrone acetate, 19.46g laurocaprone, 106.69g propylene glycol, 8.77g lauric acid, 16.27g polyethylene glycol 400, 229.39g polyacrylate and Mix 62.31g of ethyl acetate, stir evenly, and place until the bubbles disappear; 2) Coat the mixture obtained in step 1) on the anti-adhesive layer with a thickness of 0.10mm, and place it in an oven to dry at a temperature of 80°C, drying time is 60 mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com