Acetyl isovaleryl tylosin amide, preparation method and application
A technology of acetylisovaleryl tylosin amine and compound, which is applied in the field of novel acetylisovaleryl tylosin amide, and achieves the effects of simple preparation method, mild conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
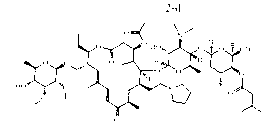
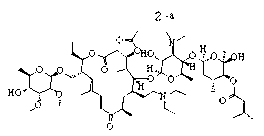
[0037] Example 1: Preparation formula 2 Shown-NR 1 R 2 =-N(C 2 h 5 ) 2 Derivatives( 2-a )
[0038] take compound 1 (416mg, 0.4mmol), diethylamine (62μl, 0.6mmol) was dissolved in 5ml of methanol, after heating to 40°C, formic acid (31μl, 1.5mmol) was added, after constant temperature reaction for 8h, after the system was concentrated, 20ml of water was added Dissolve the residue, then adjust the pH with aqueous sodium hydroxide solution until no precipitation occurs, then filter with suction, dry and weigh the filter cake to obtain the compound 2-a (350mg), yield 80.61%, off-white solid.
Embodiment 2
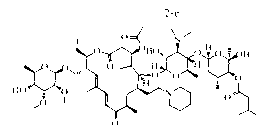
[0039] Example 2: Preparation formula 2 Shown-NR 1 R 2 =-N(n-C 4 h 9 ) 2 Derivatives( 2-b )
[0040] take compound 1 (416mg, 0.4mmol), di-n-butylamine (101μl, 0.6mmol) was dissolved in 5ml of methanol, after heating to 60°C, sodium borohydride (23mg, 1.5mmol) was added, and after constant temperature reaction for 8h, the system was concentrated, Add 20ml of water to dissolve the residue, then adjust the pH with aqueous sodium hydroxide solution until no precipitation occurs, then filter with suction, dry and weigh the filter cake to obtain the compound 2-b (320mg), yield 70.08%, off-white solid.
Embodiment 3
[0041] Embodiment 3: Preparation formula 2 Shown-NR 1 R 2 =-NC 5 h 10 (tetrahydropyrrolyl) derivatives ( 2-d )
[0042] take compound 1 (416mg, 0.4mmol), tetrahydropyrrole (50μl, 0.6mmol) was dissolved in 5ml ethanol, after heating to 60°C, formic acid (31μl, 1.5mmol) was added, after constant temperature reaction for 8h, the system was concentrated, and 20ml of water was added Dissolve the residue, then adjust the pH with aqueous sodium hydroxide solution until no precipitation occurs, then filter with suction, dry and weigh the filter cake to obtain the compound 2-d (300mg), yield 69.22%, off-white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com