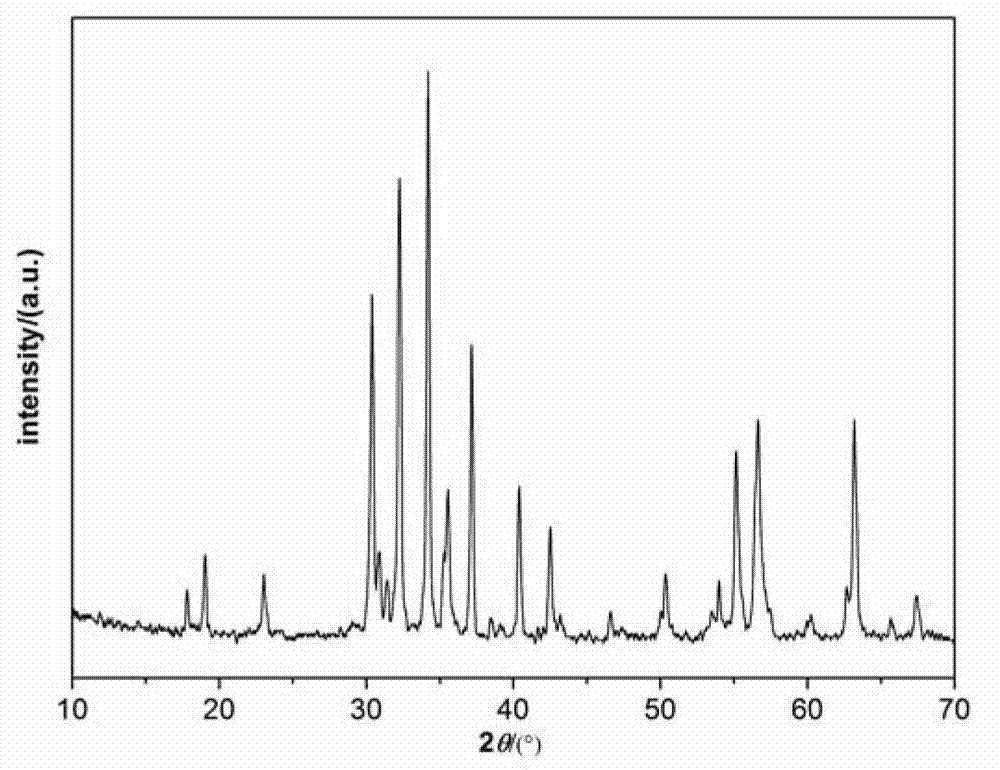
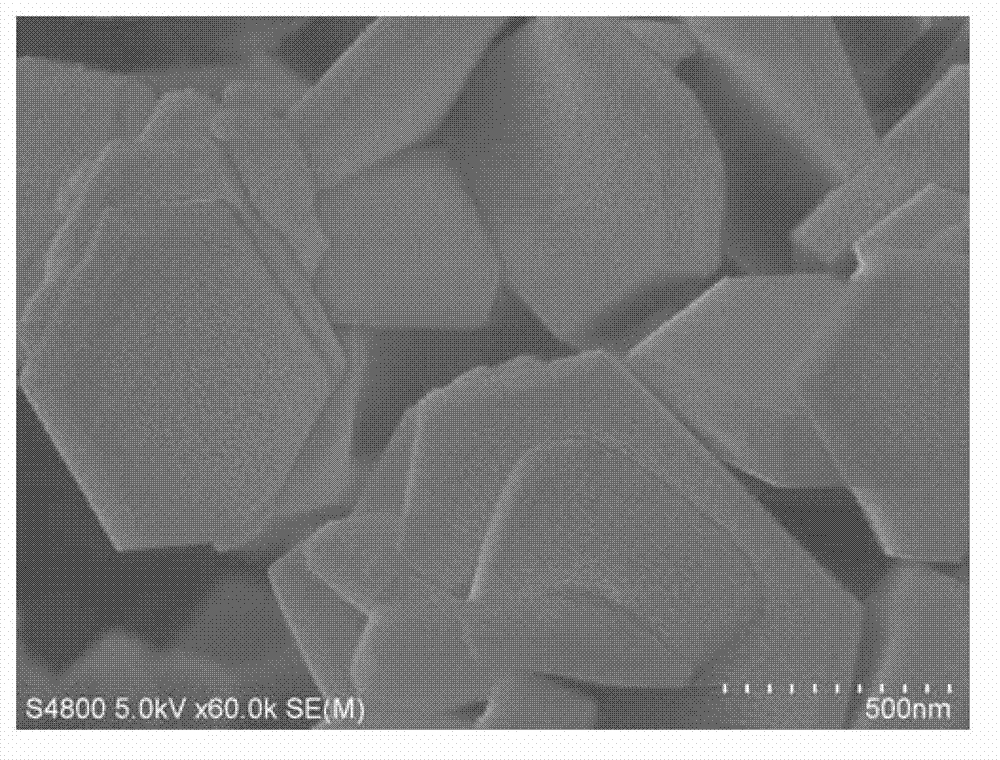
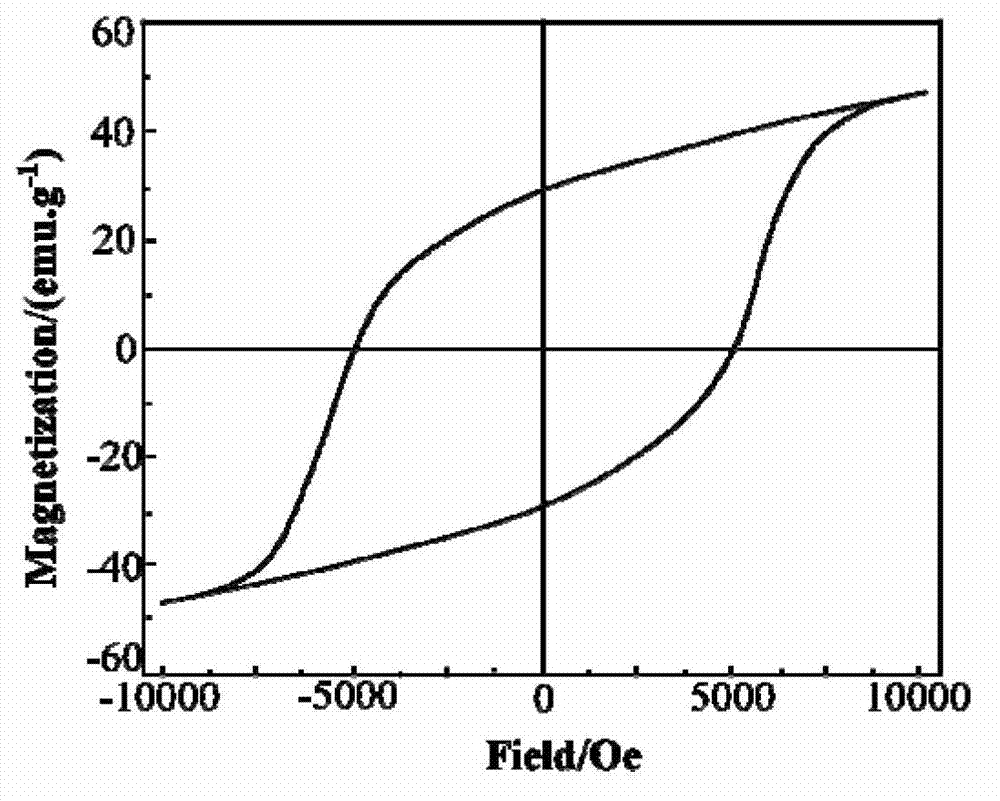
Preparation method for Zr-Mn-Co multi-doped barium ferrite wave-absorbing material
A technology of barium ferrite and wave-absorbing materials, which is applied in the field of preparation of electromagnetic wave-absorbing materials, and can solve problems such as poor dispersion and easy agglomeration of synthetic powders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] The preparation method of Zr-Mn-Co multi-component doped barium ferrite wave-absorbing material of the present invention specifically comprises the following steps:
[0019] 1) Choose commercially available Zr(OH) 4 , MnCO 3 、Co 2 o 3 Dissolved in nitric acid solution with a mass fraction of 56-58%, and continuously stirred in a water bath at 60-80°C, it is configured to be made of Zr(NO 3 ) 4 , Mn(NO 3 ) 2 , Co(NO 3 ) 3 To the formed transparent mixed solution A, add deionized water to control the total molar concentration of the three metal ions in the solution A within the range of 0.01-0.1 molar concentration. The Zr(OH) 4 , MnCO 3 、Co 2 o 3 According to the molar ratio of Zr:Mn:Co of 0.625:1:1, the amount of nitric acid is just enough to dissolve Zr(OH) 4 , MnCO 3 、Co 2 o 3 It is appropriate.
[0020] 2) According to MnCO 3 The molar ratio of EDTA to ethylenediaminetetraacetic acid (EDTA) is 1:2~8. Weigh EDTA, add it to solution A, stir well to di...
Embodiment 1
[0027] 1) Choose commercially available Zr(OH) 4 , MnCO 3 、Co 2 o 3 Dissolved in a nitric acid solution with a mass fraction of 56-58%, and continuously stirred in a water bath at 60°C, it is configured to be made of Zr(NO 3 ) 4 , Mn(NO 3 ) 2 , Co(NO 3 ) 3 For the transparent mixed solution A formed, add deionized water to make the total molar concentration of the three metal ions in the solution A controlled within the range of 0.1 molar concentration. The Zr(OH) 4 , MnCO 3 、Co 2 o 3 According to the molar ratio of Zr:Mn:Co of 0.625:1:1, the amount of nitric acid is just enough to dissolve Zr(OH) 4 , MnCO 3 、Co 2 o 3 It is appropriate.
[0028] 2) According to MnCO 3The molar ratio of ethylenediaminetetraacetic acid (EDTA) and ethylenediaminetetraacetic acid (EDTA) is 1:2. Weigh EDTA, add it to solution A, stir it well to dissolve it, and obtain solution B.
[0029] 3) Weigh Ba(NO 3 ) 2 and Fe(NO 3 ) 3 9H 2 O, according to the first Ba(NO 3 ) 2 After ...
Embodiment 2
[0034] 1) Choose commercially available Zr(OH) 4 , MnCO 3 、Co 2 o 3 Dissolved in a nitric acid solution with a mass fraction of 56-58%, and continuously stirred in a water bath at 70°C, it is configured to be made of Zr(NO 3 ) 4 , Mn(NO 3 ) 2 , Co(NO 3 ) 3 Formed transparent mixed solution A, adding deionized water to make the total molar concentration of the three metal ions in solution A controlled within the range of 0.08 molar concentration. The Zr(OH) 4 , MnCO 3 、Co 2 o 3 According to the molar ratio of Zr:Mn:Co of 0.625:1:1, the amount of nitric acid is just enough to dissolve Zr(OH) 4 , MnCO 3 、Co 2 o 3 It is appropriate.
[0035] 2) According to MnCO 3 The molar ratio of ethylenediaminetetraacetic acid (EDTA) and ethylenediaminetetraacetic acid (EDTA) is 1:4. Weigh EDTA, add it to solution A, stir well to dissolve it, and obtain solution B.
[0036] 3) Weigh Ba(NO 3 ) 2 and Fe(NO 3 ) 3 9H 2 O, according to the first Ba(NO 3 ) 2 After Fe(NO 3 )...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Saturation magnetization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com