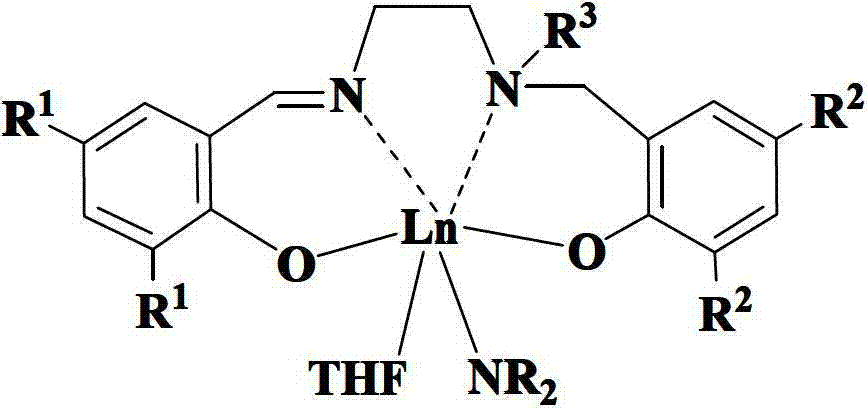
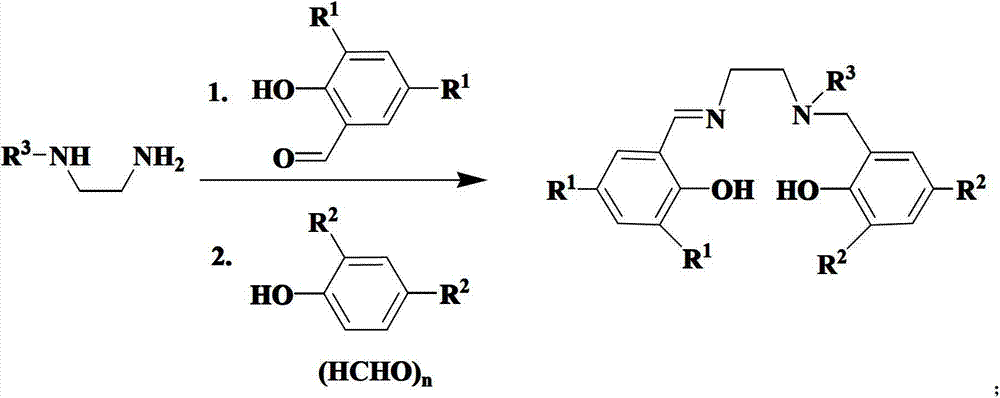
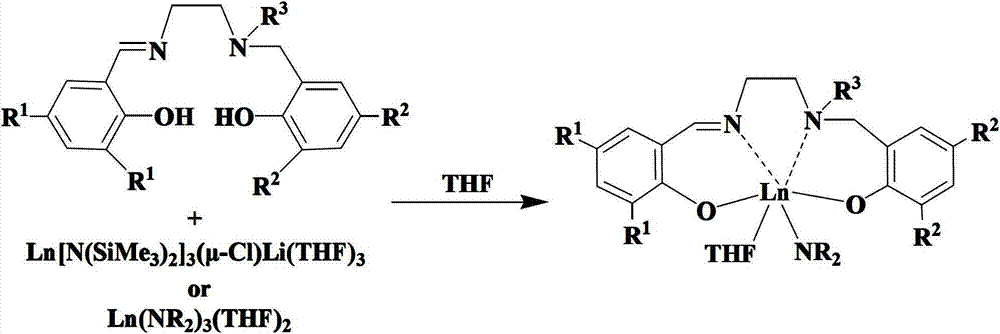
Salalen-type rare-earth metal amide as well as preparation method and application thereof
A rare earth metal and amide technology, applied in the direction of organic chemistry, can solve the problems of rare rare earth metal complexes and other problems, and achieve the effects of convenient separation and purification, clear structure and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Preparation of SalalenH 2 (R 1 =R 2 = Bu t )
[0050] Add 4.68 grams (20 mmoles) of 3,5-di-tert-butyl salicylaldehyde into a two-necked bottle with a stirring bar,
[0051] Add methanol to dissolve, drop two drops of glacial acetic acid, then add 1.8mL (20 mmol) of N-methylethylenediamine, stir for half an hour, then add 4.12 g (20 mmol) of 2,4-di-tert-butylphenol ), 0.9 g (30 mmol) of paraformaldehyde, reacted at 65-75° C. for 12 hours, a large amount of yellow solids precipitated, filtered with suction, washed the filter cake with methanol, and drained, the yield was 80%. NMR data: 1 H NMR (400MHz, CDCl 3 ): 8.37(s, 1H), 7.38(d, 1H), 7.20(d, 1H), 7.07(d, 1H), 6.82(d, 1H), 3.77(t, 2H), 3.74(s, 2H) , 2.84(t, 2H), 2.38(s, 3H), 1.44(s, 9H), 1.37(s, 9H), 1.30(s, 9H), 1.27(s, 9H).
Embodiment 2
[0052] Example two: (Salalen)Y[N(SiMe 3 ) 2 ](THF)
[0053] Y[N(SiMe 3 ) 2 ] 3 (μ-Cl)Li(THF) 3 (2.89 mmol) THF solution was added to SalalenH 2 (1.47 grams, 2.89 mmol) in the tetrahydrofuran solution, the system turns light yellow transparent solution from bright yellow, 25 DEG C of stirring reaction about 2 hours, remove tetrahydrofuran, add 20 milliliters of normal hexanes and extract twice, centrifuge and remove a little precipitation, The supernatant was transferred, concentrated, and left overnight at room temperature, and 1.91 g of light yellow crystals were precipitated, with a yield of 80%. Elemental analysis: C, 62.09; H, 9.43; N, 5.25; Y, 10.98; C 43 h 76 N 3 o 3 Si 2 Theoretical values for Y: C, 62.36; H, 8.25; N, 5.07; Y, 10.74. 1 H NMR (400MHz, C 6 D. 6 , 25°C): δ7.71(d, J=2.5Hz, 1H, ArH), 7.50(d, J=2.4Hz, 1H, ArH), 7.26(s, 1H, N=CHAr), 6.96(d , J=2.3Hz, 1H, ArH), 6.89(d, J=2.5Hz, 1H, ArH), 4.37(d, J=12.7Hz, 1H, ArCH 2 N), 3.58 (br, 4H, α-CH 2 T...
Embodiment 3
[0054] Embodiment three: (Salalen)Sm[N(SiMe 3 ) 2 ](THF)
[0055] Sm[N(SiMe 3 ) 2 ] 3 (μ-Cl)Li(THF) 3 (2.82 mmol) THF solution was added to SalalenH 2 (1.44 g, 2.82 mmol) in THF solution, the system changed from bright yellow to yellow solution, and stirred at 25°C for about 2 hours, removed THF, added 20 ml of n-hexane for extraction twice, centrifuged to remove a little precipitate, and the supernatant After transferring, concentrating, overnight at room temperature, 1.86 g of yellow crystals were precipitated, with a yield of 74%. Elemental analysis: C, 58.36; H, 8.80; N, 4.07; Sm, 16.79;
[0056] C 43 h 76 N 3 o 3 Si 2 Theoretical values for Sm: C, 58.06; H, 8.61; N, 4.72; Sm, 16.90. Infrared absorption spectrum data: 2960s, 2900s, 2869s, 1620s, 1540m, 1470s, 1440s, 1410m, 1360mm, 1310m, 1250m, 1200w, 1160m, 928m, 877m, 839s, 742w, 617w.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com