Preparation method of chitosan 6-OH immobilized cyclodextrin derivative and application thereof in H2O2 detection fluorescent biosensor
A chitosan derivative, 6-OH technology, applied in fluorescence/phosphorescence, material excitation analysis, etc., can solve the problems of limited solubility of derivatives, difficult to dissolve, and limited applications.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
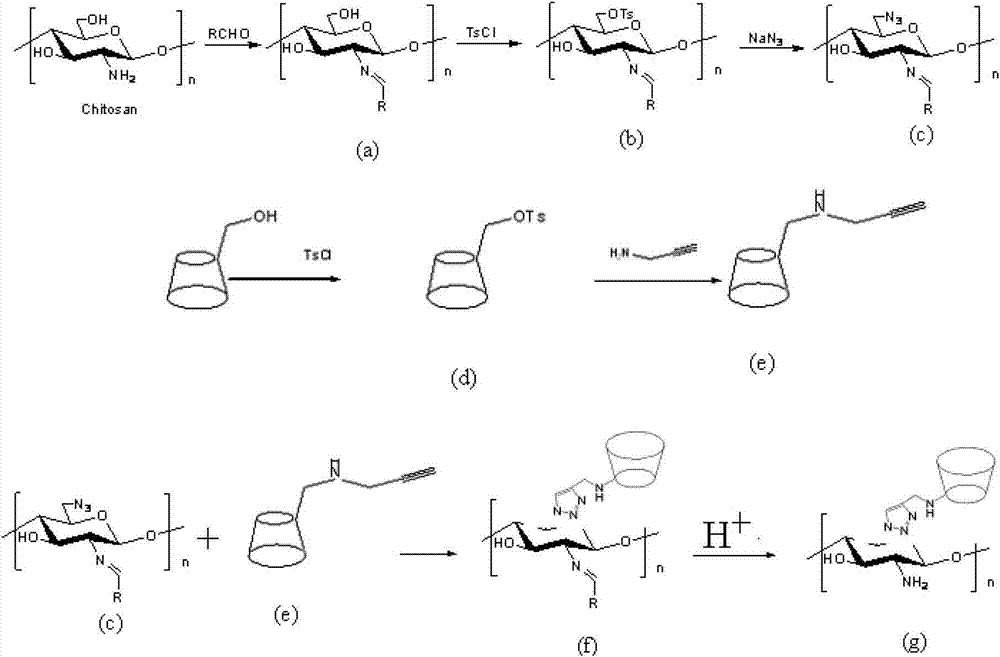
[0078] Embodiment 1 (preparation of chitosan 6-OH immobilized cyclodextrin derivatives)
[0079]1 part of chitosan was weighed and dissolved in 200 parts of acetic acid / water solution with a mass concentration of 1%. 2 parts of phthalic anhydride dissolved in 50 parts of ethanol were added dropwise to the reaction system, and the reaction was stirred at 50°C for 5 hours. Then add methanol to precipitate the resulting chitosan derivatives, wash with ethanol more than 2 times to remove excess phthalic anhydride compounds, and then vacuum dry at 60°C to obtain phthalic anhydride-protected chitosan derivatives a .
[0080] 1 part of phthalic anhydride-protected chitosan derivative a is dissolved in 200 parts of DMSO, and 30 parts of chloroform solution of p-toluenesulfonyl chloride are added thereto, wherein the mass concentration of p-toluenesulfonyl chloride is 20% , dripped in 10 minutes, reacted at 30°C for 3 hours, filtered with suction to obtain a light yellow solid, washe...
Embodiment 2
[0086] Embodiment 2 (preparation embodiment of chitosan 6-OH immobilized cyclodextrin derivative)
[0087] Utilize the click chemical reaction of the azide derivative c of chitosan prepared in specific example 1 and the monoalkynylation derivative e of cyclodextrin to prepare phthalic anhydride chitosan immobilized cyclodextrin derivative Object f:
[0088] Weigh 1 part of the azide derivative c of chitosan, dissolve it in 10 parts of DMAc, and weigh 0.03 parts of CuSO 4 ·5H 2 O was dispersed in 5 parts of ethanol, adding 0.03 part of sodium ascorbate and 0.05 part of water to promote dissolution, mixed the solution, weighed 4 parts of monoalkynylated derivative e, added the above solution, stirred and reacted at 60°C for 5 hours, Stop the reaction, add acetonitrile to precipitate, wash with water twice, and vacuum-dry the filter cake at 60° C. to obtain phthalic anhydride chitosan immobilized cyclodextrin derivative f.
[0089] 1 part of obtained phthalic anhydride-based c...
Embodiment 3
[0090] Embodiment 3 (use chitosan 6-OH immobilized cyclodextrin derivative of the present invention to prepare fluorescent biosensor detection material and be used for H 2 o 2 detection)
[0091] The chitosan-immobilized cyclodextrin derivative obtained in Example 1 was dissolved in DMF, wherein the concentration of the chitosan-immobilized cyclodextrin derivative in the solution was 0.025 mol / L. The fluorescent probe coumarin was added to it. In the reaction, the molar ratio of chitosan immobilized cyclodextrin derivative to coumarin was 1:1. The reaction was refluxed and stirred in a 40°C oil bath for 4 hours. After the reaction is completed, the clathrate is precipitated with deionized water, the coumarin that has not been clathrated is washed away with ether, and dried to obtain the chitosan 6-OH immobilized cyclodextrin derivative clathrate fluorescent probe coumarin product. The inclusion rate of coumarin was measured to be 39.2%.
[0092] The chitosan 6-OH immobil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com