Mature human beta-defensin-2 (HBD-2) and preparation method thereof
A β-defensin, a mature technology, applied in the field of gene recombination expression and genetic engineering, can solve the problems that the purity of mature HBD-2 cannot be guaranteed, it is not suitable for large-scale preparation, and the health of experimenters is unfavorable, so as to achieve stable expression, convenient source, The effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1. Construction of engineered bacteria producing recombinant human β defensin-2 prepropeptide
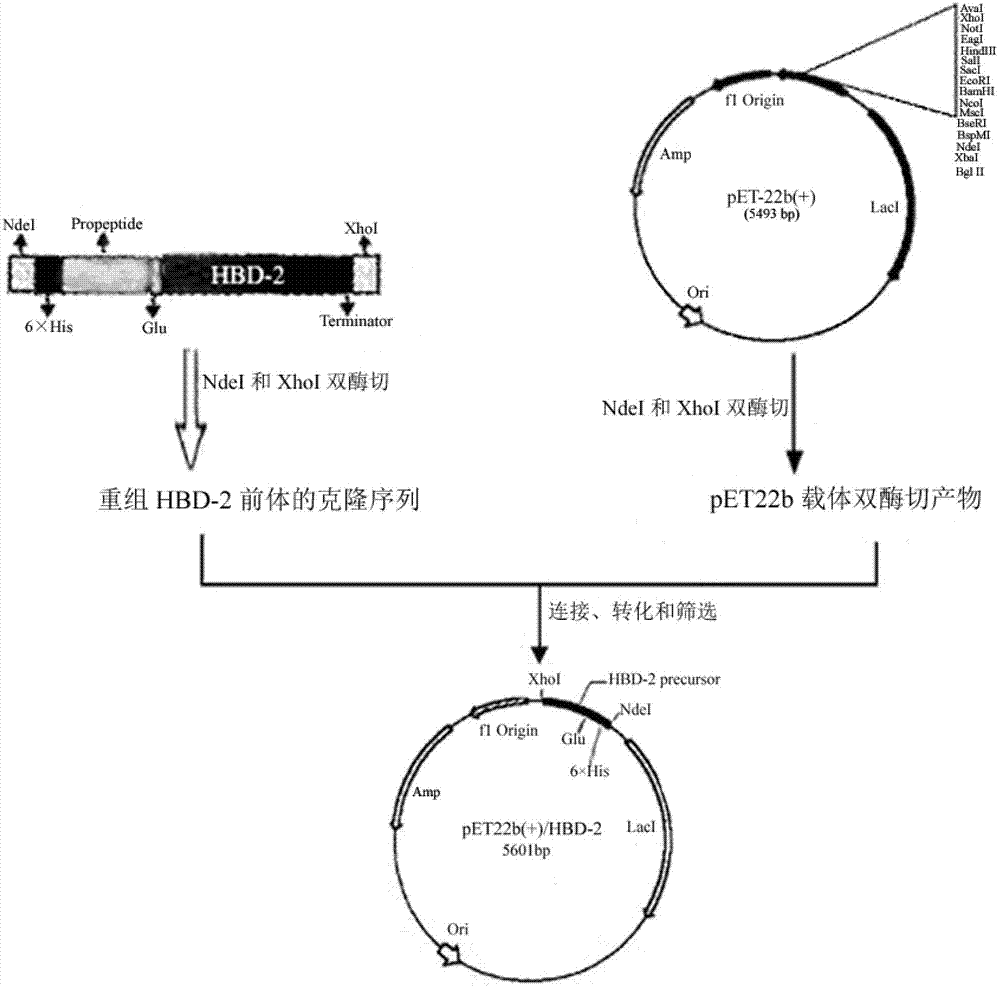
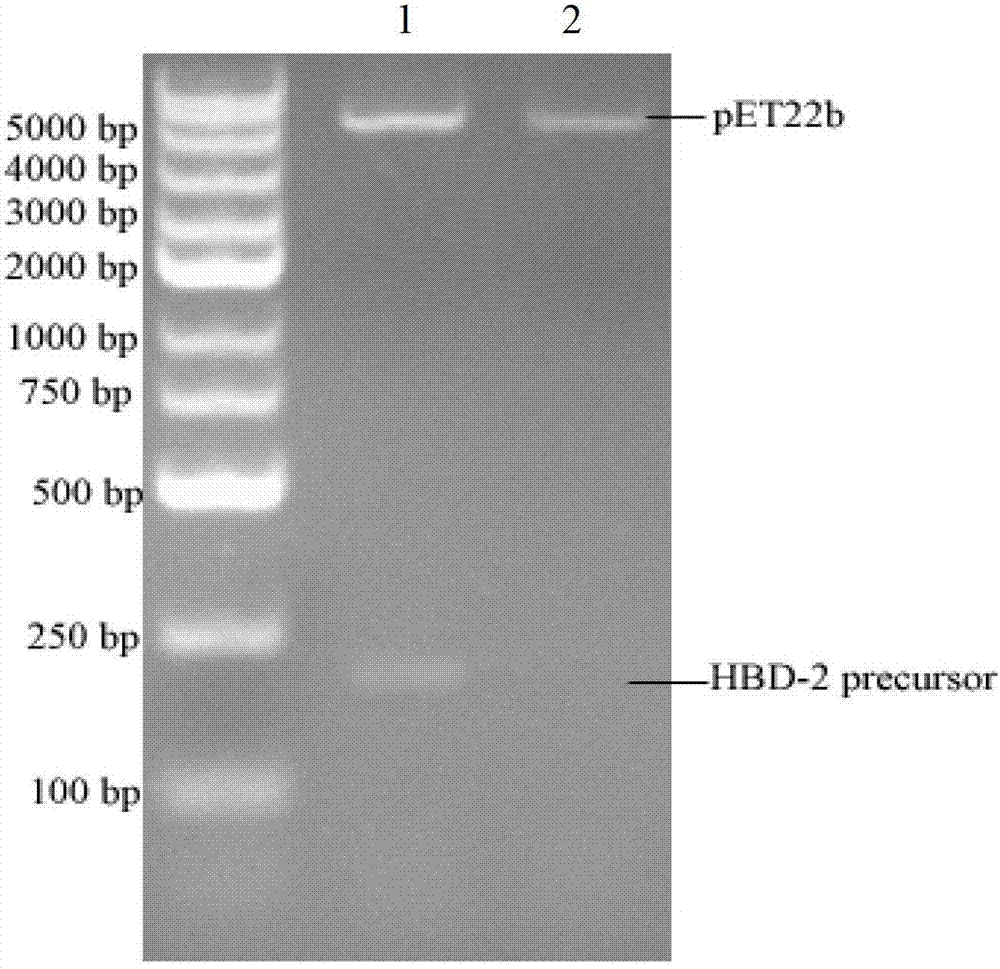
[0041] By introducing a glutamate residue between the encoding human β defensin-2 propeptide and the mature sequence as a recognition site for glutamate-specific endopeptidase, and fusing a histidine tag at the N-terminus, introducing a termination at the C-terminus After being digested with NdeI and XhoI, it is connected to the expression vector pET22b(+) to realize the construction of the recombinant plasmid pET22b-HBD-2 prepropeptide. The specific construction process is as follows: figure 1 Shown.
[0042] 1. Design and synthesis of target gene fragments and primers
[0043] According to the human β defensin-2 gene sequence (GenBank ID: AY155577), GAA was introduced as a glutamate codon between the mature sequence gene and its propeptide gene fragment, and the new recombinant HBD-2 prepropeptide gene sequence (see SEQ ID NO: 3) was synthesized by Shanghai Jierui Biological Engi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com