Lithium vanadate anode material and preparation method thereof
A positive electrode material, lithium vanadate technology, applied in the direction of battery electrodes, electrical components, circuits, etc., can solve the problem that the rate performance needs to be improved, and achieve the effects of fast capacity decay, improved cycle performance, and stable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Dissolve 0.5 g of vanadium acetylacetone in 30 mL of dimethylformamide solution and continue to stir for 5 hours. The resulting clear solution is transferred to a 30-50 mL Teflon-lined stainless steel reactor, and sealed. Heated at 150° C. for 10 hours, then cooled to room temperature. The resulting black precipitate was washed several times with distilled water, and the resulting precipitate was dried.
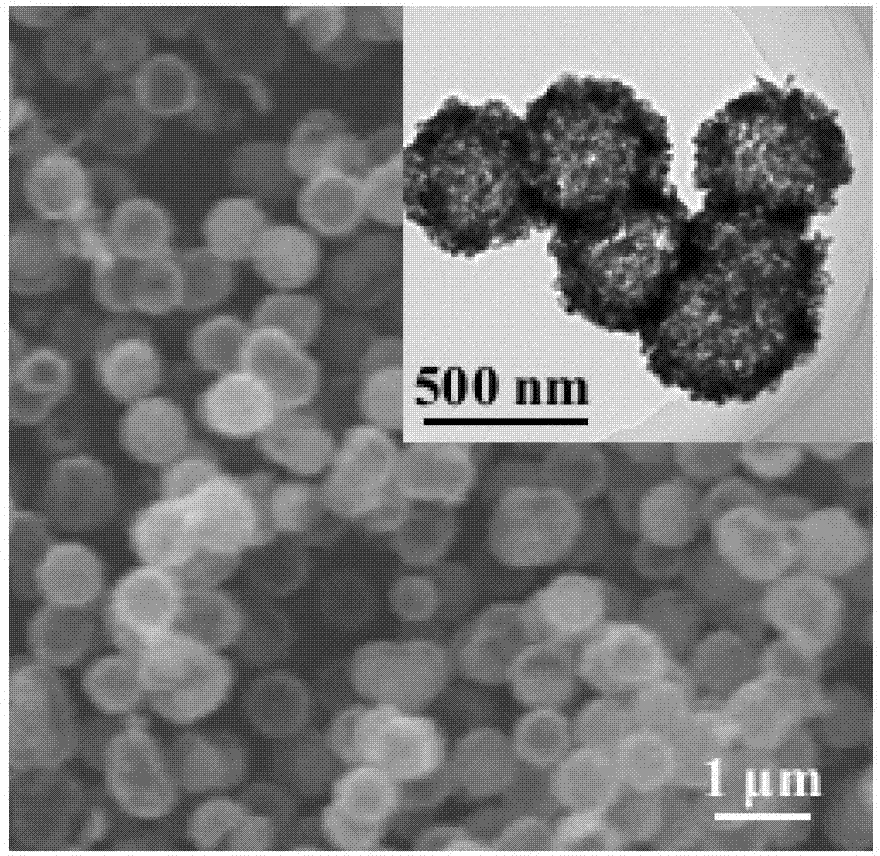
[0033] The synthesized product is V of the hollow structure of the present invention. 2 o 3 Materials, SEM images, TEM images such as figure 1 shown. It can be seen that the particle size of the obtained product is relatively uniform and the particle size is small; at the same time, it can be seen that the sample has a hollow structure and good particle dispersion.
[0034] The above obtained V 2 o 3 Precursor and LiOH·H 2 O was compounded at a molar ratio of 1.5:1.05, and after being uniformly mixed in the solution, the product was first dried at 80°C, and then...
Embodiment 2
[0041] Dissolve 0.5 g of vanadium acetylacetone in 30 mL of dimethylformamide solution and continue to stir for 5 hours. The resulting clear solution is transferred to a 30-50 mL Teflon-lined stainless steel reactor, and sealed. Heated at 150° C. for 5 hours, then cooled to room temperature. The resulting black precipitate was washed several times with distilled water, and the resulting precipitate was dried.
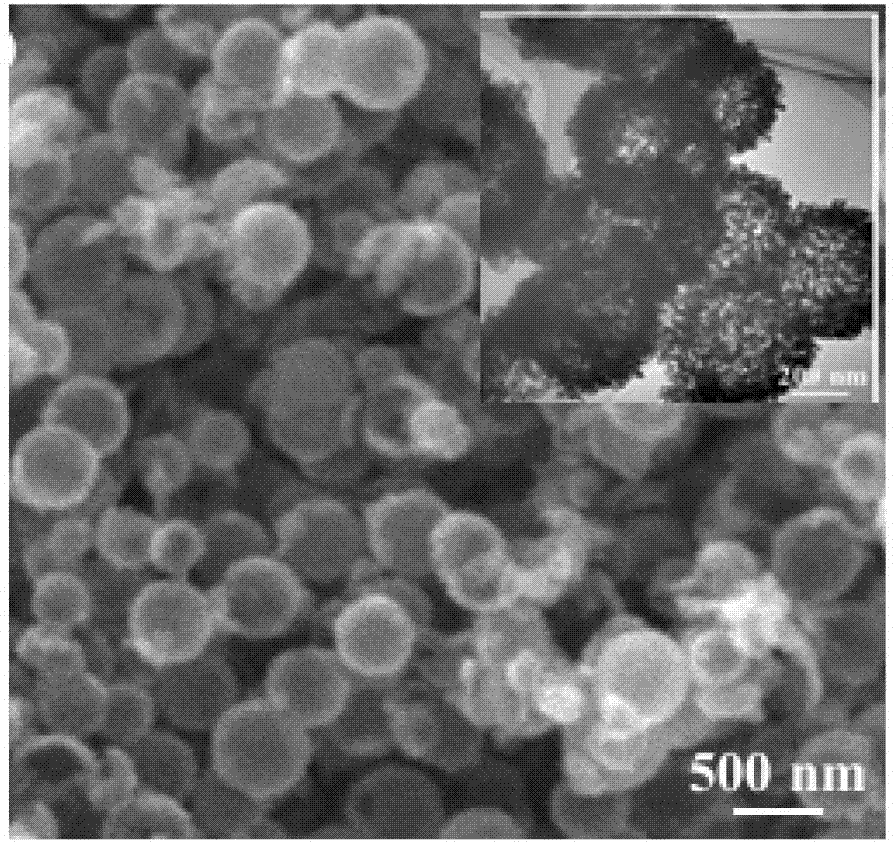
[0042] The above obtained V 2 o 3 Precursor and LiOH·H 2 O is compounded according to a molar ratio of 1.5:1.05. After mixing evenly in the solution, the product is first dried at 80°C, and then sintered at 450°C for 10 hours to obtain the final product. The particle size of the obtained material is about 420nm, the thickness of the hollow spherical shell is about 170nm, the pore diameter of the small hole of the shell is about 2.10nm, the pore diameter of the large hole is about 10nm, and the specific surface area of the material is about 62.75m 2 / g
Embodiment 3
[0044] Dissolve 0.5 g of vanadium acetylacetone in 30 mL of dimethylformamide solution and continue to stir for 5 hours. The resulting clear solution is transferred to a 30-50 mL Teflon-lined stainless steel reactor, and sealed. Then heated at 150°C for 8 hours, then cooled to room temperature. The resulting black precipitate was washed several times with distilled water, and the resulting precipitate was dried.
[0045] The above obtained V 2 o 3 Precursor and LiOH·H 2 O is compounded according to a molar ratio of 1.5:1.05. After mixing evenly in the solution, the product is first dried at 80°C, and then sintered at 450°C for 10 hours to obtain the final product. The particle size of the obtained material is about 400nm, the thickness of the hollow spherical shell is about 120nm, the diameter of the small hole in the shell is about 3.60nm, the diameter of the large hole is about 25nm, and the specific surface area of the material is about 82.87m 2 / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com