Splitting method for racemic 2-benzene propanoic acid
A technology for phenylpropionic acid and racemization, applied in the field of separating L-2-phenylpropionic acid and D-2-phenylpropionic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Mix n-hexane: ethyl acetate: phosphate buffer containing hydroxypropyl-β-cyclodextrin (prepared with 0.1mol / L phosphate buffer solution, pH=2.6, containing 0.1mol / L hydroxypropyl -β-cyclodextrin, with a substitution degree of 7.5) was configured in a separatory funnel at a volume ratio of 5:5:10, shaken up and allowed to stand to separate layers. After equilibrating for a period of time, the upper and lower phases were separated, the organic phase was used as the stationary phase, and the aqueous phase was used as the mobile phase.
[0041] (2) Weigh 40 mg of racemic 2-phenylpropanoic acid and dissolve it in 6 ml of water phase, and make a sample solution after dissolving for use.
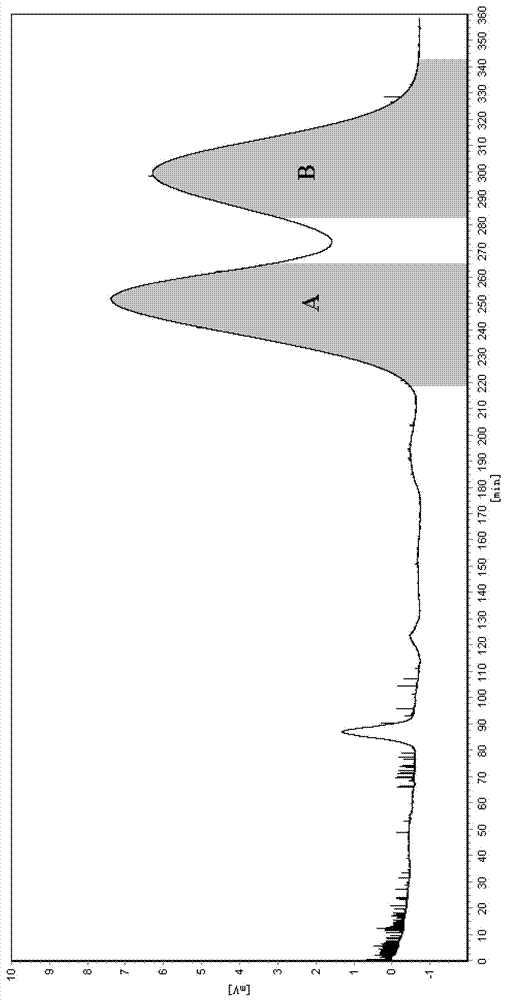
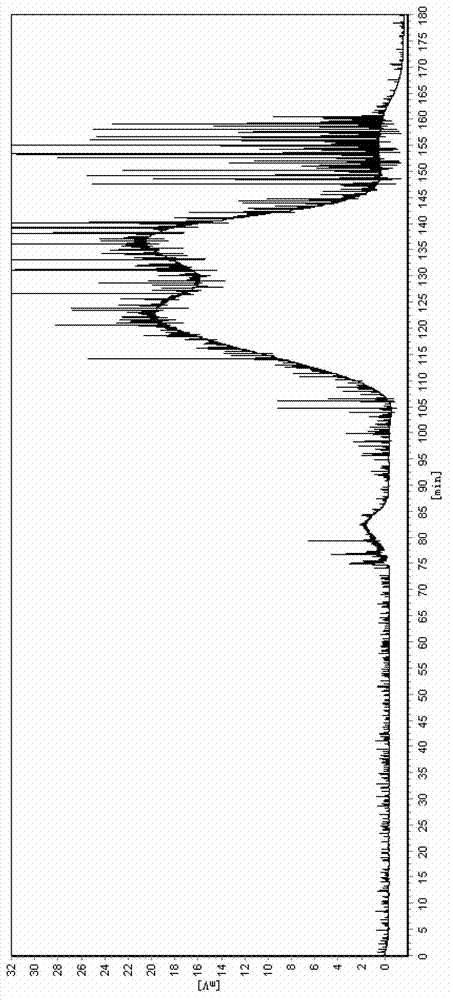
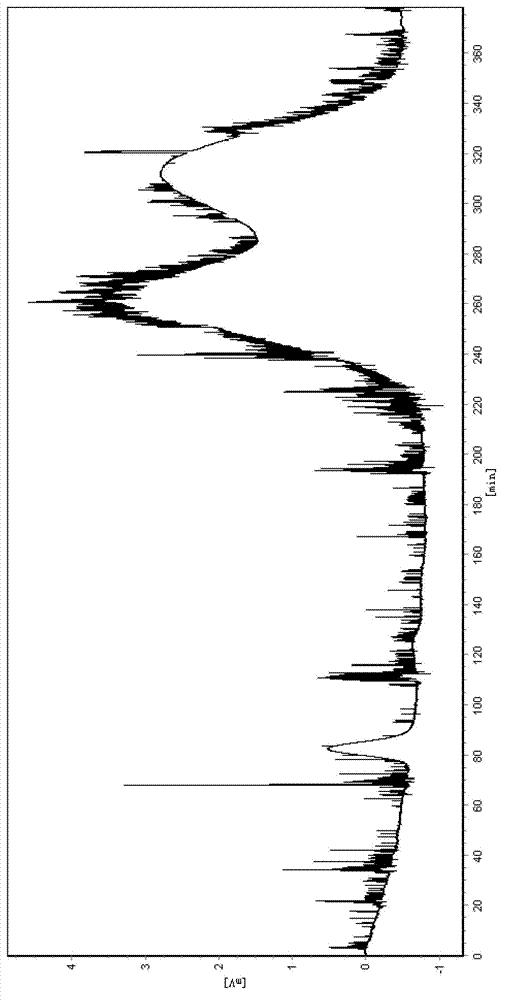
[0042] (3) Use a semi-preparative high-speed countercurrent chromatograph (Shanghai Tongtian Biotechnology Co., Ltd., instrument model TBE-300A) to resolve racemic 2-phenylpropionic acid: the volume of the separation column is 300ml, and the countercurrent chromatographic The separation...
Embodiment 2
[0045] (1) Mix n-hexane: ethyl acetate: phosphate buffer containing hydroxypropyl-β-cyclodextrin (prepared with 0.1mol / L phosphate buffer solution, pH=2.6, containing 0.1mol / L hydroxypropyl -β-cyclodextrin, the degree of substitution is 7.5) was configured in a separating funnel according to the volume ratio of 8:2:10, shake well and then stand to separate layers. After equilibrating for a period of time, the upper and lower phases were separated, the organic phase was used as the stationary phase, and the aqueous phase was used as the mobile phase.
[0046] (2) Weigh 40 mg of racemic 2-phenylpropanoic acid and dissolve it in 6 ml of water phase, and make a sample solution after dissolving for use.
[0047] (3) Use a semi-preparative high-speed countercurrent chromatograph (Shanghai Tongtian Biotechnology Co., Ltd., instrument model TBE-300A) to resolve racemic 2-phenylpropionic acid: the volume of the separation column is 300ml, and the countercurrent chromatographic The sep...
Embodiment 3
[0050] (1) Mix n-hexane: ethyl acetate: phosphate buffer containing hydroxypropyl-β-cyclodextrin (prepared with 0.1mol / L phosphate buffer solution, pH=3.2, containing 0.1mol / L hydroxypropyl -β-cyclodextrin, degree of substitution 7.5) was configured in a separatory funnel at a volume ratio of 5:5:10, shaken well and allowed to stand to separate layers. After equilibrating for a period of time, the upper and lower phases were separated, the organic phase was used as the stationary phase, and the aqueous phase was used as the mobile phase.
[0051] (2) Weigh 40mg of racemic 2-phenylpropionic acid and dissolve it in 6ml of water phase, and make a sample solution after dissolution.
[0052] (3) Use a semi-preparative high-speed countercurrent chromatograph (Shanghai Tongtian Biotechnology Co., Ltd., instrument model TBE-300A) to resolve racemic 2-phenylpropionic acid, and the volume of the separation column is 300ml. Before sample injection, the countercurrent chromatographic sep...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com