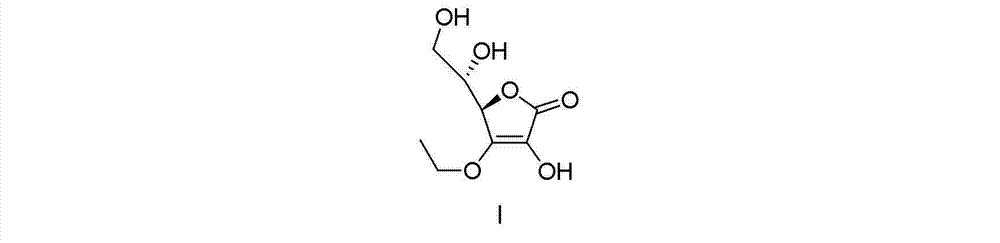
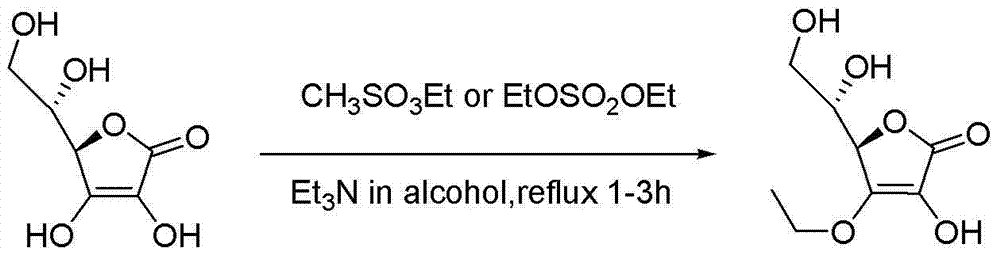
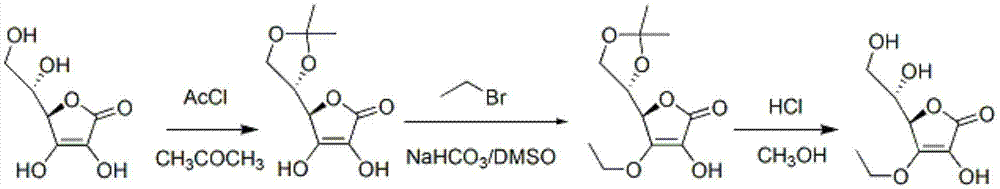
Synthesizing method of vitamin C ethyl ether
A synthesis method and vitamin technology, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of physical injury of production workers, prolonged production sections, unfavorable environmental protection, etc., achieve high equipment utilization, reduce material transfer, supply Good stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] A synthetic method of vitamin C ethyl ether, comprising the steps of:
[0043] (1) (R)-3,4-dihydroxy-5-[(S)-2,2-dimethyl-1,3-dioxolan-4-yl]furan-2(5H)-one preparation of
[0044] ①In a 30L reaction flask, under mechanical stirring and nitrogen protection, add 3.42 kg vitamin C, 2.22 kg acetone dimethyl acetal and 11.23 kg dimethyl sulfoxide, heat to 30°C and stir;
[0045] ② Add 33.4 g of catalyst p-toluenesulfonic acid and keep it warm for 180 minutes;
[0046] ③The external temperature was 40°C, and methanol and a small amount of unreacted acetone dimethyl acetal were distilled off under reduced pressure. Obtain the DMSO solution of the product of step (1). Go directly to the next reaction.
[0047] After detection, the product is (R)-3,4-dihydroxy-5-[(S)-2,2-dimethyl-1,3-dioxolan-4-yl]furan-2(5H) - Ketone, HPLC purity ≥ 97%, mp.=208-210°C. 1 HNMR (400MHz, DMSO-d 6 ) δ (ppm): 1.23 (6 H, s), 3.86-3.88 (1H, dd, J=6.3, 8.4Hz), 4.05-4.09 (1 H, dd, J=7.0, 8.4Hz), 4....
Embodiment 2
[0058] A synthetic method of vitamin C ethyl ether, comprising the steps of:
[0059] (1) (R)-3,4-dihydroxy-5-[(S)-2,2-dimethyl-1,3-dioxolan-4-yl]furan-2(5H)-one preparation of
[0060] ①In a 30L reaction flask, under mechanical stirring and nitrogen protection, add 3.42 kg vitamin C, 2.22 kg acetone dimethyl acetal and 11.23 kg dimethyl sulfoxide, heat to 30°C and stir;
[0061] ② Add 48.7 g catalyst pyridinium p-toluenesulfonate and keep warm for 180 minutes;
[0062] ③The external temperature was 40°C, and methanol and a small amount of unreacted acetone dimethyl acetal were distilled off under reduced pressure. Obtain the DMSO solution of the product of step (1). Go directly to the next reaction.
[0063] After detection, the product is (R)-3,4-dihydroxy-5-[(S)-2,2-dimethyl-1,3-dioxolan-4-yl]furan-2(5H) - Ketone, HPLC purity ≥ 97%, mp.=208-210°C.
[0064] (2) (R)-4-ethoxy-3-hydroxy-5-[(S)-2,2-dimethyl-1,3-dioxolan-4-yl]furan-2(5H ) - Preparation of ketones
[0065]...
Embodiment 3
[0074] Other conditions are the same as in Example 1, the difference is that (R)-4-ethoxy-3-hydroxyl-5-[(S)-2,2-dimethyl-1,3 In the step of -dioxolan-4-yl]furan-2(5H)-one, 3.289 kg of bromoethane was put into the step and reacted for 8 hours. The total yield of the two steps was 65% (calculated as vitamin C).
[0075] After detection, the product is (R)-4-ethoxy-3-hydroxy-5-[(S)-2,2-dimethyl-1,3-dioxolan-4-yl]furan-2 (5H)-ketone, HPLC purity ≥94%, mp. =104-106℃.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com