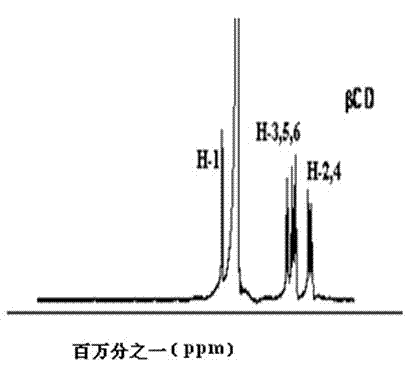
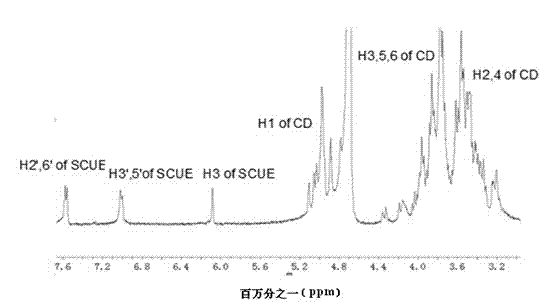
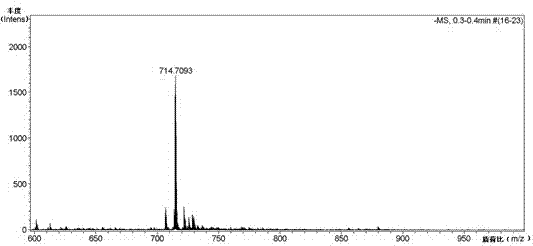
Scutellarein aglycone prodrug and preparation method thereof
A technology of baicalein aglycone and scutellarin is applied in the field of scutellarin aglycone prodrug and its preparation, and achieves the effects of mild reaction conditions, simple operation and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: The preparation of mono(6-amino-6-deoxy)-β-cyclodextrin bonded scutellarein prodrug is as follows:
[0046] Add 0.25 g (0.87 mmol) of scutellarein aglycone, 64 mL (0.87 mmol) of 37% formaldehyde solution, and 0.986 g (0.87 mmol), anhydrous N,N-dimethylformamide 15mL and catalytic amount 0.87mL (0.0087mmol) concentrated hydrochloric acid, stir evenly, under the protection of inert gas nitrogen, heat up to 50°C, react for 10h, after the reaction, 60 The reaction solution was evaporated to dryness under reduced pressure at ℃, the residue was fully dissolved in 3 mL of water, filtered, 300 mL of acetone was added dropwise to the filtrate, filtered, the precipitate was collected, and vacuum-dried at 50 °C for 24 hours to obtain 6-scutellarein bonded The crude product of β-cyclodextrin, the yield is 1.095g, and the yield is 88%. It is purified and refined by LH-20 gel column, washed with water and methanol-water, and the same components are combined to obtain mon...
Embodiment 2
[0048] Example 2: The preparation of mono(6-ethylenediamino-6-deoxy)-α-cyclodextrin bonded scutellarein prodrug is as follows:
[0049] Add 0.25g (0.87mmol) of scutellarein aglycone, 78mg (2.51mmol) of paraformaldehyde, and 2.046g (1.74 mmol), 15 mL of anhydrous dimethyl sulfoxide, and a catalytic amount of 1.13 mg (0.0087 mmol) of boron trifluoride diethyl ether, stirred evenly, under the protection of inert gas argon, heated to 25 ° C, and reacted for 48 h. After the reaction, 60 The reaction solution was evaporated to dryness under reduced pressure at ℃, the residue was fully dissolved in 3 mL of water, filtered, 300 mL of acetone was added dropwise to the filtrate, filtered, the precipitate was collected, and vacuum-dried at 50 °C for 24 hours to obtain 6-scutellarein bonded β-cyclodextrin crude product, the yield is 1.072g, the yield is 84%. Purify and refine with LH-20 gel column, wash with water and methanol-water, combine the same components to obtain mono(6-ethylen...
Embodiment 3
[0050] Example 3: The preparation of mono(6-diethylenediamino-6-deoxy)-γ-cyclodextrin bonded scutellarein aglycone prodrug is as follows:
[0051] Add 0.25g (0.87mmol) of scutellarein aglycone, 52mg (1.74mmol) of paraformaldehyde, and 3.183g of mono(6-diethylenediamine-6-deoxy)-γ-cyclodextrin into the reaction flask in sequence ( 2.51mmol), water 15mL and catalyst 1.74mL (0.0174mmol) concentrated hydrochloric acid, stir evenly, under the protection of inert gas argon, heat up to 75°C, react for 24h, after the reaction, evaporate the reaction solution under reduced pressure at 60°C, The residue was fully dissolved in 3 mL of water, filtered, 300 mL of acetone was added dropwise to the filtrate, filtered, the precipitate was collected, and vacuum-dried at 50°C for 24 hours to obtain the crude product of 6-scutellarein bonded β-cyclodextrin with a yield of 1.127g, the yield is 85%. Purify and refine with LH-20 gel column, wash with water and methanol-water, combine the same co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com