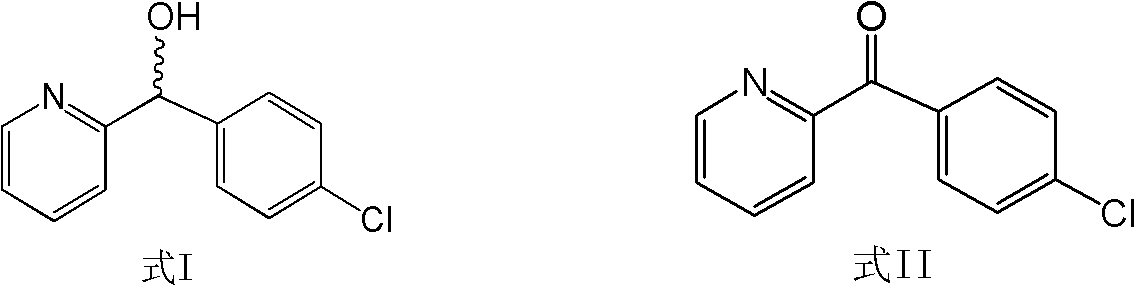
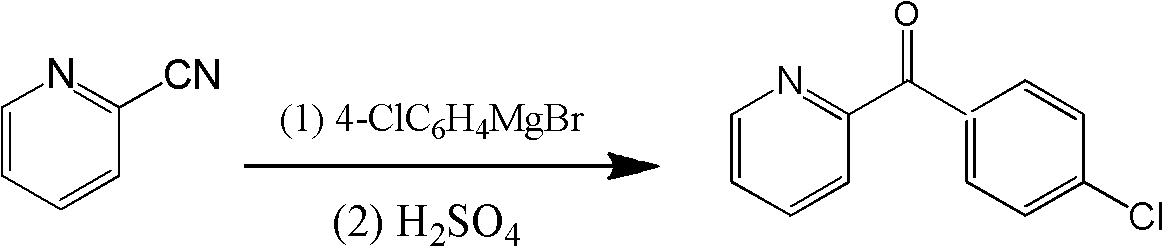Method for directionally synthesizing (4-chlorphenyl)-(pyridine-2-base)-methanol
A technology for directional synthesis and compound, applied in the direction of organic chemistry, etc., can solve the problems of many steps, complicated operation and high cost, and achieve the effects of mild reaction conditions, simple and easy method, and high product yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] 2. The preparation method of chiral reducing agent BINAL-H is as follows:
[0033]
[0034] 3. The purity of the chiral product in R or S configuration (4-chlorophenyl)-(pyridin-2-yl)-methanol is determined by HPLC, and detected by a chiral chromatographic column. The detection conditions are as follows:
[0035] Testing instrument: LC-10ATVP
[0036] Chromatographic column: chiral column Chiral OD (Daicel)
[0037] Mobile phase: hexane-ethanol (95:5)
[0038] Detection wavelength: 254nm
[0039] Injection concentration and volume: 100ug / ml, 20ul
[0040] Column temperature: 35°C
[0041] Flow rate: 0.75ml / min
Embodiment 1
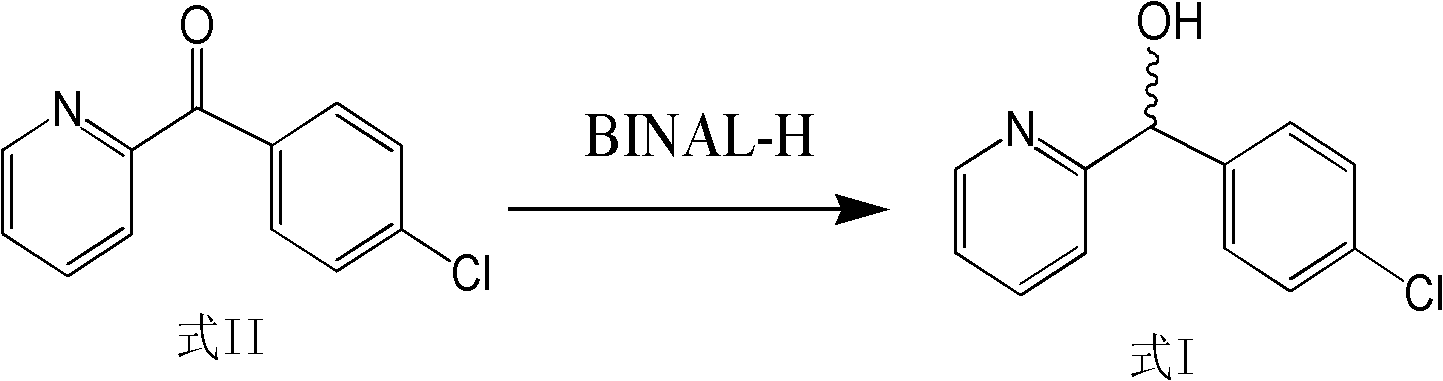
[0043] Take 87.6g (0.4mol) of (4-chlorophenyl)-(pyridin-2-yl)-methanone (Formula II), dissolve it in 200mL of dichloromethane, and add it dropwise to In 1000mL of dichloromethane containing 0.8mol (S)-BINAL-H, keep the reaction at -100~-80°C, and detect the end point of the reaction by thin-layer chromatography (the developing solvent is methanol: chloroform: acetic acid 7: 2: 1 ). After the reaction is complete, add 300 mL of ethanol to the reaction solution, adjust the pH to 4.5-5.5 with 1N hydrochloric acid, add 500 mL of saturated saline, extract with ether, dry the extract with magnesium sulfate, concentrate, and recrystallize with ethyl acetate-petroleum ether. 63.3 g of (S)-(4-chlorophenyl)-(pyridin-2-yl)-methanol was obtained, with a yield of 72.3% and a purity of 86.2%.
Embodiment 2
[0045] Take 87.6 g (0.4 mol) of (4-chlorophenyl)-(pyridin-2-yl)-methanone (Formula II), dissolve it in 200 mL of ether, and add it dropwise to 3.2 mol(R)-BINAL-H in 1000mL ether solution, kept at -60~-50°C for reaction, and detected the end point of the reaction by thin-layer chromatography (developing solvent: methanol: chloroform: acetic acid 7:2:1). After the reaction is complete, add 500 mL of methanol to the reaction solution, adjust the pH to 4.5-5.5 with 1N hydrochloric acid, add 500 mL of saturated saline, extract with ether, dry the extract with magnesium sulfate, concentrate, and recrystallize with ethyl acetate-petroleum ether. 72.8 g of (R)-(4-chlorophenyl)-(pyridin-2-yl)-methanol was obtained, with a yield of 82.3% and a purity of 95.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com