Doripenem intermediate solvate
A technology for doripenem and intermediates, applied in the field of ethanolate of doripenem intermediates, can solve the problems of low safety, long preparation cycle, toxicity, etc., and achieve the effects of easy storage, low toxicity and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Preparation of the ethanolate of the doripenem intermediate shown in formula 2.
[0056] Compound 4 (33.6kg, 63.1mol) was dissolved in methanol (140kg), concentrated sulfuric acid (15.8kg) was added dropwise, heated to 65°C and refluxed for 2.5h, then cooled to 25°C, and concentrated to 110mL under reduced pressure. Pour the concentrated solution into a mixed solution of ethyl acetate (225kg) and water (250kg), separate the liquids, wash the organic phase three times with 5% NaCl (175kg), and back-extract the aqueous phase with ethyl acetate (90kg) each time , combined the organic phases and dried over anhydrous sodium sulfate for 1 h. Concentrated under reduced pressure to 70kg, added to compound 3 (30.0kg, 50.5mol) and DMF (143kg) mixture, cooled to 0°C under nitrogen protection, added DIPEA dropwise, stirred at 0-5°C for 18h.
[0057] After the reaction, 2 times the volume of ethanol was added to the reaction solution, stirred at room temperature for 4 h...
Embodiment 2
[0063] Example 2: Preparation of an intermediate of doripenem represented by formula 2 (refer to Document 1).
[0064] Compound 4 (33.6g, 0.0631mol) was dissolved in methanol (140g), concentrated sulfuric acid (15.8g) was added dropwise, heated to 65°C and refluxed for 2.5h, then cooled to 25°C, and concentrated to 110mL under reduced pressure. The concentrated solution was poured into a mixed solution of ethyl acetate (225g) and water (250g), separated, the organic phase was washed three times with 5% NaCl (175g), and each aqueous phase was back-extracted with ethyl acetate (90g) , combined organic phases, concentrated under reduced pressure to 70g, added to compound 3 (30.0g, 0.0505mol) and DMF (143g) mixture, cooled to 0°C under nitrogen protection, added DIPEA dropwise, stirred at 0-5°C for 18h, Then the reaction solution was introduced into ethyl acetate (200g) and water (225mL), the phases were separated, and the organic phase was successively washed with 0.7%HCl (153g),...
Embodiment 3
[0065] Example 3: Preparation of the ethanolate of the doripenem intermediate shown in formula 2.
[0066] Dissolve 10 g of the crude doripenem intermediate obtained in Example 2 in 90 mL of ethyl acetate, then add 360 mL of ethanol, stir at 10°C for 8 hours, filter with suction, wash with ethanol, and dry in vacuo at 25°C for 6 hours to obtain a pale yellow solid 8.8 g, yield 88%, HPLC purity 98.448%.
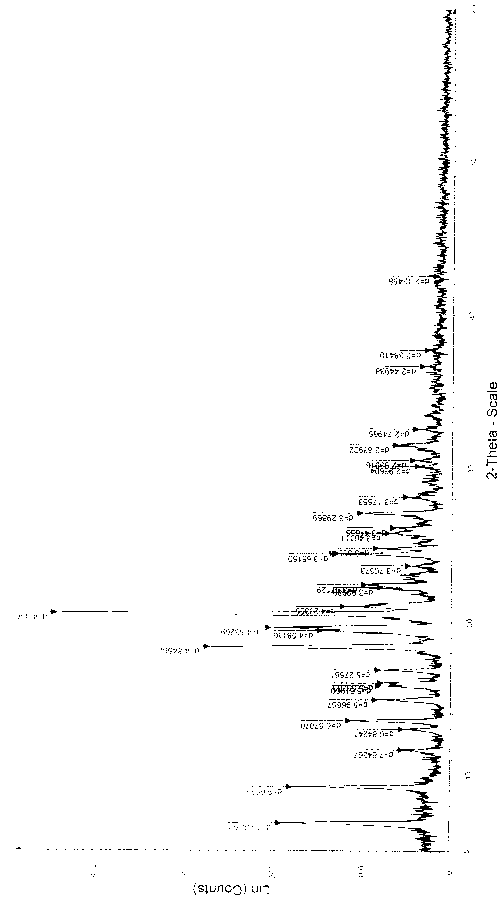
[0067] The gained solid is subjected to X-ray powder diffraction, and the main characteristic peaks are as follows (see attached figure 2 ):
[0068] .
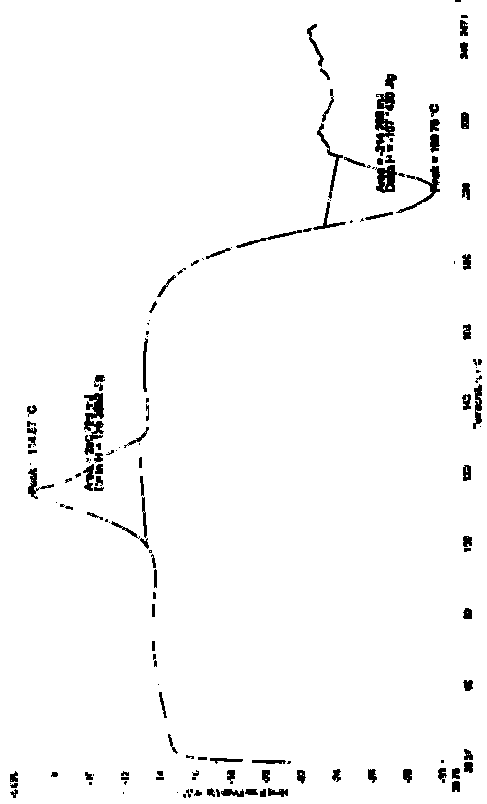
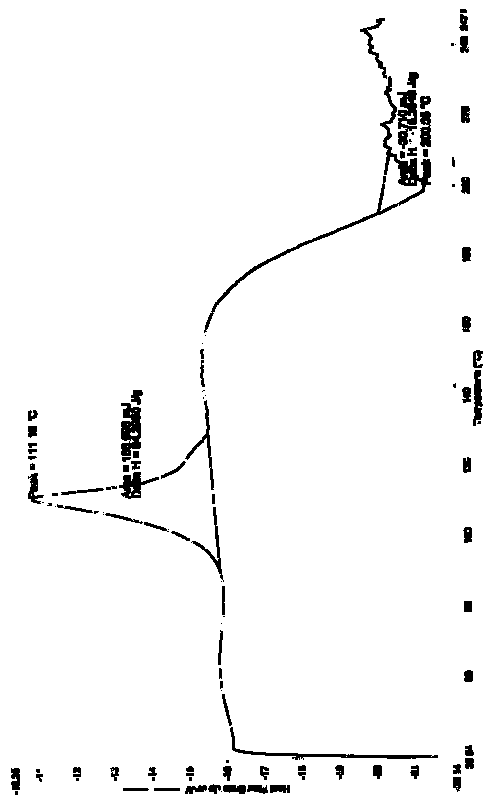
[0069] The resulting solid was subjected to a DSC test, showing an endothermic peak at 114.57°C and an exothermic peak at 199.76°C (see attached Figure 4 ).
[0070] The ethanol content of the obtained solid was determined by gas phase, and the molar ratio of ethanol to the doripenem intermediate shown in Formula 2 was 0.25:1.
[0071] The resulting solid was 1 H-NMR (500 MHz, Acetone-d6) test, from the obtained sp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com