Method of preparing iron oxide yellow by using titanium dioxide by-product
A technology of iron oxide yellow and by-products, applied in the directions of iron oxide, iron oxide/iron hydroxide, iron sulfate, etc., can solve the problems of the color effect of iron oxide yellow, mixed cylindrical seeds, less seeds, etc. Dispersion, high tinting strength, good hue effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
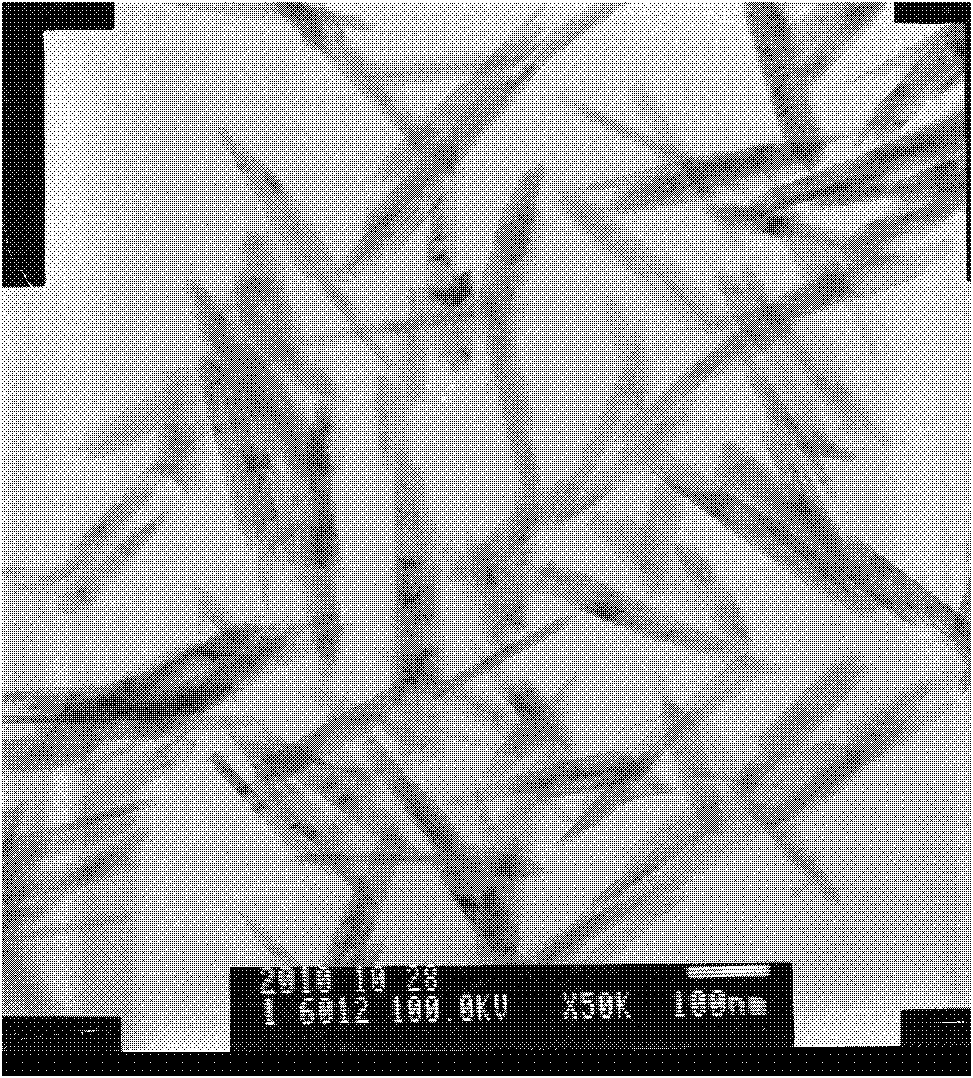
Image
Examples
Embodiment 1
[0048] (1) Refining of ferrous sulfate
[0049] Under normal temperature, utilize titanium dioxide by-product ferrous sulfate preparation concentration to be 500ml of ferrous sulfate heptahydrate solution of 350g / L, adjust the pH value of this solution with sulfuric acid to be 1, carry out titanium hydrolysis treatment to titanium dioxide by-product ferrous sulfate; Add 26g iron powder to reduce Fe in the ferrous sulfate heptahydrate solution after titanium hydrolysis treatment 3+ And adjust the pH value to 6.0, reduce the magnesium, manganese, zinc ions to obtain the precipitation of magnesium, manganese, zinc simple substance, add 0.09g polyacrylamide to the solution after titanium hydrolysis treatment and magnesium, manganese, zinc precipitation treatment Flocculant, stirring, settling for 5 hours and then filtering to remove the product of titanium hydrolysis and the precipitation of magnesium, manganese and zinc, and the filtrate is the refined ferrous sulfate solution; ...
Embodiment 2
[0060] (1) Refining of ferrous sulfate
[0061] At 50°C, 500ml of ferrous sulfate heptahydrate solution with a concentration of 370g / L was prepared by using ferrous sulfate, a by-product of titanium dioxide, and the pH value of the solution was adjusted to 1.5 with sulfuric acid, and titanium dioxide was hydrolyzed by ferrous sulfate, a by-product of titanium dioxide. treatment; then add 28g iron powder to reduce Fe in the ferrous sulfate heptahydrate solution after titanium hydrolysis treatment 3+ And adjust the pH to 6.1, reduce the magnesium, manganese and zinc ions to obtain the precipitation of magnesium, manganese and zinc, and when the solution is cooled to room temperature, add 0.09g polyacrylamide flocculant, stirred, allowed to settle for 2 hours, and then filtered to remove titanium hydrolysis products and magnesium, manganese, and zinc precipitates, and the filtrate was the refined ferrous sulfate solution;
[0062] (2)Fe(OH) 2 generation
[0063] Get 375ml of t...
Embodiment 3
[0072] (1) Refining of ferrous sulfate
[0073] At 60°C, 500ml of ferrous sulfate heptahydrate solution with a concentration of 400g / L was prepared by using ferrous sulfate, a by-product of titanium dioxide, and the pH value of the solution was adjusted to 2.0 with sulfuric acid, and titanium dioxide was hydrolyzed by ferrous sulfate, a by-product of titanium dioxide. treatment; then add 30g iron powder to reduce Fe in the ferrous sulfate heptahydrate solution after titanium hydrolysis treatment 3+ And adjust the pH to 6.0, reduce the magnesium, manganese and zinc ions to obtain the precipitation of magnesium, manganese and zinc, and when the solution is cooled to room temperature, add 0.1g of polyacrylamide flocculant, stirred, left to settle for 1.5h, and then filtered to remove titanium hydrolysis products, magnesium, manganese, and zinc precipitates, and the filtrate was the refined ferrous sulfate solution;
[0074](2)Fe(OH) 2 generation
[0075] Get 375ml of the refin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com