Method for synthesizing 3,3,3-trifluoro methyl propionate
A technology of methyl trifluoropropionate and a synthesis method, applied in the field of chemistry, can solve the problems of few reaction steps, low reaction yield, complicated post-processing and the like, and achieves less loss of intermediate products, wide sources and simple post-processing Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
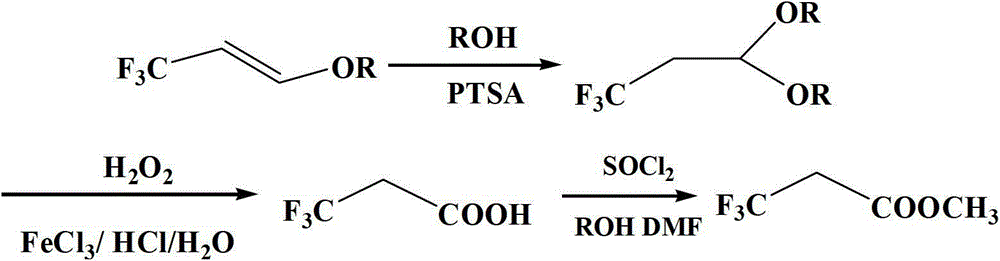
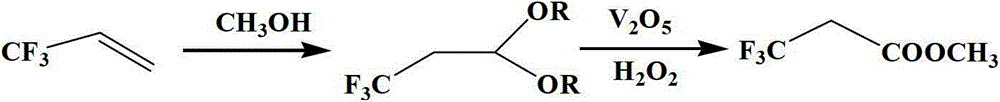
[0021] According to the technical scheme of the present invention, the synthetic method of 3,3,3-trifluoromethyl propionate of the present invention specifically comprises the steps:
[0022] (1) Put methanol, Pd / C catalyst, and pro-oxidant in the reaction kettle, feed 3,3,3-trifluoropropene and oxygen, heat up to 70°C~120°C, react for 8h~12h, cool, and put The reaction solution is filtered, and the filtrate is used in the next step, wherein, the mass ratio of 3,3,3-trifluoropropene: Pd / C catalyst: methanol: pro-oxidant is 1: 1.4~2.5: 0.02~0.2: 0.04~0.4; Pd / The palladium content of C catalyst is 5% of catalyst gross mass, and prooxidant is cupric chloride or ferric chloride;
[0023] (2) Add the filtrate, protonic acid and pro-oxidant obtained in step (1) into a three-necked flask, raise the temperature to 60°C~80°C, add 30% hydrogen peroxide dropwise, and then react at 60°C~80°C for 4h~ 8h; the reaction solution was filtered and distilled, and the fraction at 70°C~73°C was ...
Embodiment 1
[0029] (1) Put 20g of methanol, 0.26g of Pd / C catalyst, and 1.33g of copper chloride in an autoclave, and feed 13g of raw material 3,3,3-trifluoropropene at -50°C, and then pass After adding 1MPa oxygen, heat up to 120°C with stirring, react for 8 hours, cool to room temperature, slowly release the gas in the system, open the kettle, filter the reaction solution, separate and remove the catalyst, and the filtrate is used in the next step.
[0030] (2) Put 25g of the reaction solution obtained in step (1) into a three-necked flask, add 1.9g of hydrochloric acid and 0.5g of vanadium pentoxide, raise the temperature to 60°C, and slowly add 9g of hydrogen peroxide with a mass fraction of 30% under stirring In the reaction flask, react for 6h. The reaction solution was filtered and distilled at atmospheric pressure, and the fraction at 70~73°C was collected to obtain 11.9 g of methyl 3,3,3-trifluoropropionate with a purity of 97%. The total yield of the two steps is 59.8%.
Embodiment 2
[0032] (1) Put 20g of methanol, 0.5g of Pd / C catalyst, and 1.33g of ferric chloride in a high-pressure reactor, inject 13g of raw material 3,3,3-trifluoropropene at -50°C, and inject 1MPa After oxygen, the temperature was raised to 100°C with stirring, and the reaction was carried out for 24 hours. After the reaction, cool to room temperature, slowly release the gas in the system, open the kettle to discharge, filter the reaction solution, separate and remove the catalyst, and the filtrate is used in the next step.
[0033] (2) Put 25g of the filtrate obtained in step (1) into a three-necked flask, add 2.2g of hydrochloric acid and 1.5g of vanadium pentoxide, heat up to 80°C, and slowly add 18g of hydrogen peroxide with a mass fraction of 30% under stirring In the reaction flask, react for 4h. The reaction solution was filtered and distilled at atmospheric pressure, and the fraction at 70~73°C was collected to obtain 11.9 g of methyl 3,3,3-trifluoropropionate with a purity of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com