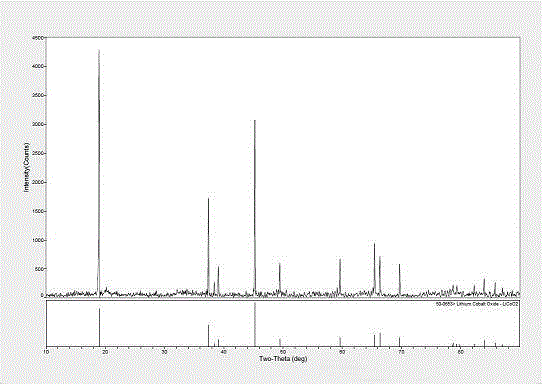
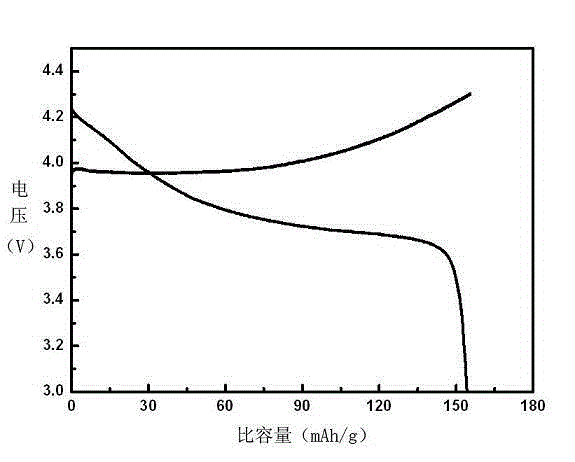
Preparation method of lithium cobalt oxide
A technology of lithium cobalt oxide and lithium salt, applied in the field of positive electrode materials of lithium ion batteries, can solve the problems of uneven content distribution, large particle size of lithium cobalt oxide, precipitation of lithium element, etc. less agglomeration effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Weigh a certain amount of lithium salt and take the total molar weight of lithium salt as the basis. The lithium salt is lithium carbonate (M 碳酸锂 : 74 g / mol), weigh 74g (1mol), and measure the water-soluble organic matter according to the proportion that the total molar weight of water-soluble organic matter is 50% of the total molar weight of lithium salt, and the water-soluble organic matter is citric acid (M 柠檬酸 : 192 g / mol), 96 g was measured.
[0039] (2) After mixing citric acid and lithium carbonate, add water with 10% (9.6 g) of citric acid mass, and ball mill at 100 rpm for 5 hours to obtain a pre-reaction slurry.
[0040] (3) Add cobalt-containing materials and 30% (57.6 g) water of citric acid mass to the pre-reaction slurry with a molar ratio of Li:Co of 0.95:1, and ball mill at a speed of 50 rpm for 12 hours to obtain the reaction Slurry, wherein, cobalt-containing material is cobalt carbonate (M 碳酸钴 : 119 g / mol), the added amount is 251 g.
[0041] ...
Embodiment 2
[0053] (1) Weigh a certain amount of lithium salt and take the total molar weight of lithium salt as the basis. The lithium salt is lithium hydroxide (M 氢氧化锂 : 24 g / mol), weigh 24g (1mol), and measure the water-soluble organic matter according to the proportion that the total molar weight of water-soluble organic matter is 60% of the total molar weight of lithium salt. The water-soluble organic matter is citric acid and glucose (M 柠檬酸 : 192 g / mol, M 葡萄糖 : 180 g / mol), the molar weights of citric acid and glucose were 0.3 mol each, and a total of 111.6 g was measured.
[0054] (2) After mixing citric acid, glucose and lithium hydroxide, add 15% (16.74g) of water to the total mass of citric acid and glucose, and ball mill at 200rpm for 3 hours to obtain a pre-reaction slurry.
[0055] (3) Add cobalt-containing materials and 40% (44.64 g) water of the total mass of citric acid and glucose to the pre-reaction slurry with a molar ratio of Li:Co of 1:1, and ball mill for 8 hours at ...
Embodiment 3
[0061] (1) Weigh a certain amount of lithium salt and take the total molar weight of lithium salt as the basis. Lithium salt is lithium carbonate and lithium hydroxide (M 碳酸锂 : 74 g, M 氢氧化锂 : 24 g / mol), weigh 74 g lithium carbonate, 24 g lithium hydroxide, the total molar mass is 2 mol, measure the water-soluble organic matter according to the ratio of the total molar mass of water-soluble organic matter to 70% of the total molar mass of lithium salt, water-soluble organic matter citric acid, glucose and oxalic acid (M 柠檬酸 : 192 g / mol, M 葡萄糖 : 180 g / mol , M 草酸 : 90 g / mol), in which, 0.5 mol of citric acid and glucose, 0.4 mol of oxalic acid, 222 g in total.
[0062] (2) After mixing citric acid, glucose, oxalic acid with lithium carbonate and lithium hydroxide, add water which is 20% (44.4g) of the total mass of citric acid, glucose and oxalic acid, and ball mill for 2 hours at a speed of 300rpm to obtain a pre-reaction slurry material.
[0063] (3) Add cobalt-containing ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com