Preparation method of ultrafine artesunate sterile powder
The technology of artesunate and fine artesunate is applied in the field of preparation of ultra-fine artesunate sterile powder, which can solve the problems of wide product particle size distribution, narrow particle size distribution and high equipment cost, and achieves easy dissolving speed, The effect of simple and easy control and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
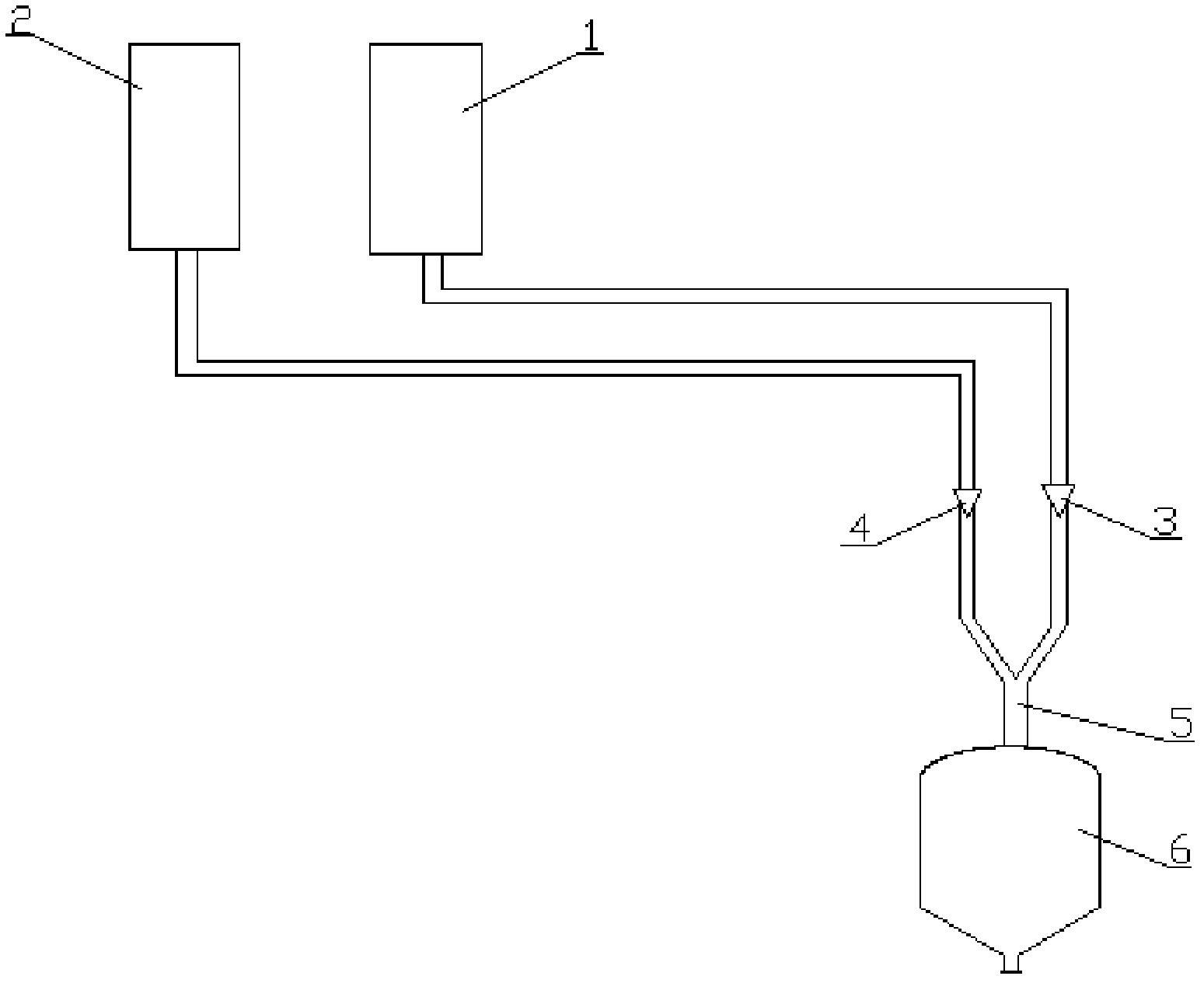
[0030] 1) Get 2g of artesunate raw drug, dissolve it in ethanol at 20°C to make a concentration of 0.05g / ml (5% of the concentration of artesunate sterile powder in ethanol under this temperature condition) About 40ml of the bulk drug solution is placed in the bulk drug solution storage tank 1, and 400ml of deionized water is placed in the anti-solvent storage tank 2;
[0031] 2) Through the bulk drug solution advection pump 3 and the anti-solvent advection pump 4, the bulk drug solution and water are pumped into the microreactor 5 substantially simultaneously through the solution inlet and the anti-solvent inlet respectively for recrystallization, and the slurry obtained by the recrystallization is produced by the microreaction The output pipeline of the device 5 is collected to the slurry storage tank 6; the flow rate of the bulk drug solution is 1ml / min, the flow rate of the deionized water is 10ml / min, and the recrystallization temperature is 10°C;
[0032] 3) Filter the s...
Embodiment 2
[0036] 1) Get 10.0g of artesunate raw drug, dissolve it in ethanol at 20°C to make a concentration of 0.2g / ml (that is, 20% of the concentration of artesunate sterile powder in ethanol under this temperature condition) About 50ml of bulk drug solution is placed in bulk drug solution storage tank 1, and 1250ml deionized water is placed in antisolvent storage tank 2;
[0037] 2) Through the bulk drug solution advection pump 3 and the anti-solvent advection pump 4, the bulk drug solution and water are pumped into the microreactor 5 substantially simultaneously through the solution inlet and the anti-solvent inlet respectively for recrystallization, and the slurry obtained by the recrystallization is produced by the microreaction The output pipeline of the device 5 is collected to the slurry storage tank 6; the flow rate of the bulk drug solution is 4ml / min, the flow rate of the deionized water is 20ml / min, and the recrystallization temperature is 15°C;
[0038] 3) The slurry obta...
Embodiment 3
[0041]1) Take 8.0kg of artesunate raw drug, dissolve it in ethanol at 20°C to make a concentration of 0.2kg / L (that is, 20% of the saturated concentration of artesunate sterile powder in ethanol under this temperature condition). %) of the crude drug solution 40L, placed in the crude drug solution storage tank 1, get 1200L deionized water and placed in the anti-solvent storage tank 2;
[0042] 2) Through the bulk drug solution advection pump 3 and the anti-solvent advection pump 4, the bulk drug solution and water are pumped into the microreactor 5 substantially simultaneously through the solution inlet and the anti-solvent inlet respectively for recrystallization, and the slurry obtained by the recrystallization is produced by the microreaction The output pipeline of the device 5 is collected to the slurry storage tank 6; the flow rate of the bulk drug solution is 4ml / min, the flow rate of the deionized water is 60ml / min, and the recrystallization temperature is 15°C;
[0043...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com