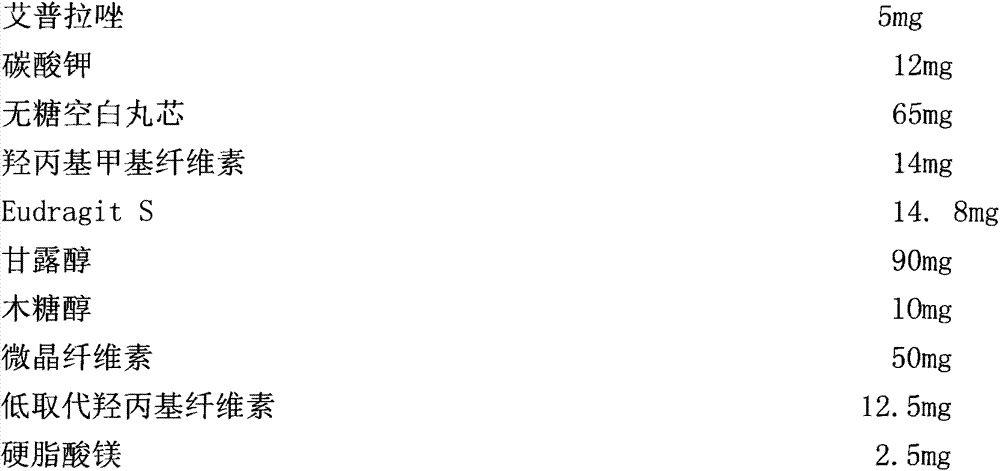A kind of ilaprazole enteric-coated orally disintegrating tablet and preparation method thereof
A technology for enteric and orally disintegrating tablets of ilaprazole, applied in the field of medicine, can solve the problem of not finding orally disintegrating tablets of ilaprazole, and achieve the effects of good taste, fast absorption and good disintegration effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1 prepares ilaprazole enteric-coated orally disintegrating tablet
[0042] Prescription 1:
[0043]
[0044] Preparation:
[0045] (1) Coating for the first time: Dissolve ilaprazole and sodium carbonate in 50ml of water, add 450ml of absolute ethanol, and place the blank pellet core on an inverted cone-shaped stainless steel with a bottom diameter of 188mm, a top diameter of 396mm, and a height of 1000mm. In the bed, the atomizing nozzle adopts a top-spraying two-stream nozzle, 200mm away from the material surface; air is used as the fluidized drying medium and the atomized gas of the material liquid. Set the frequency of the blower to 50HZ, the bed temperature of the fluidized bed to 40°C, the fluidization gas velocity to 2.0m / s, the flow velocity of the feed liquid to 0.5ml / min, and the atomizing pressure to 0.20mpa, start the fluidized bed, After the coating is complete, continue to keep warm for 10 minutes.
[0046] (2) Second coating: dissolving h...
Embodiment 2
[0058] Example 2 Stability study of ilaprazole enteric-coated orally disintegrating tablets
[0059] Influencing factor test: Ilaprazole orally disintegrating tablets with prescriptions 1 to 3 were subjected to high temperature test (placed at high temperature (60°C) for 10 days) and high humidity test (at 25°C compared to relative Humidity (90±5)% for 10 days), strong light test (10 days for illuminance of 5000±500Lx). Referring to ilaprazole orally disintegrating tablets, the storage condition of this product is to be sealed and stored in a cool and dry place.
[0060] The test results are shown in Table 2:
[0061] Table 2. Test results of influencing factors of prescription 1 ilaprazole enteric-coated orally disintegrating tablets
[0062]
[0063] Under the same conditions, the ilaprazole enteric-coated orally disintegrating tablet of prescription 2 was subjected to the experiment of influencing factors, and the experimental results were obtained.
[0064] Table 3. ...
Embodiment 3
[0070] Example 3 Pharmacological comparison experiment of ilaprazole enteric-coated orally disintegrating tablet, rabeprazole enteric-coating orally disintegrating tablet and rabeprazole sodium enteric-coating orally disintegrating tablet
[0071] Ilaprazole enteric-coated orally disintegrating tablets (prepared by using prescription 1 of Example 1 of the present invention) and rabeprazole enteric-coating orally disintegrating tablets, rabeprazole enteric-coating orally disintegrating tablets (specification: 10 mg, batch number: 008025 , Xi'an Xintong Drug Research Co., Ltd.) for comparison.
[0072] Rabeprazole enteric-coated orally disintegrating tablet: prepared by changing ilaprazole in the prescription to rabeprazole with reference to prescription 1 of Example 1 of the present invention.
[0073] pharmacological test
[0074] Effects on chronic ulcers in rats (chronic ulcers induced by acetic acid)
[0075] The specific test method is as follows:
[0076] The experimen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com