Secretin analogue, as well as preparation method and application thereof
An analog, secretin technology, applied in the direction of insulin, hormone peptides, biological testing, etc., can solve the problems of reduced biological activity, instability of natural secretin, increased transportation and storage costs, etc., to achieve improved chemical stability, The effect of reducing degradation, increasing the degree of α-helix
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation of embodiment 1 secretin analog
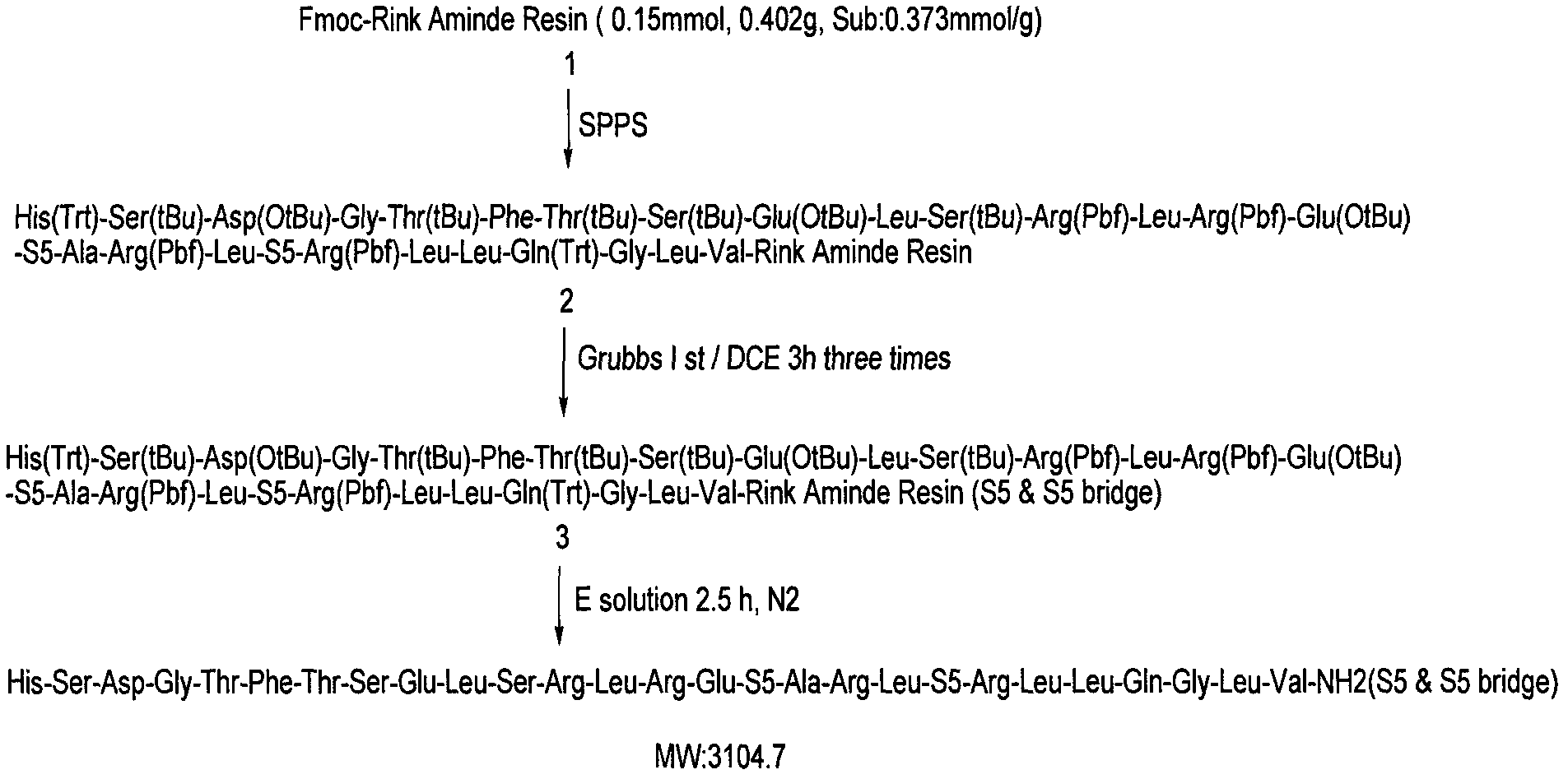
[0040] according to figure 1 In the steps shown, the amino acids are attached to the resin one by one, and then a catalyst is used to catalyze the ring closure, and then the protecting group is removed and the polypeptide is cleaved from the resin.
[0041] 1. Solid phase synthesis
[0042] Select 0.402g Rink amide linker MBHA resin (substitution degree: 0.373mmol / g) to prepare, soak the resin with DMF at room temperature for 60min, arrange the amino acids with Fmoc protection in the synthesizer according to the order of the sequence, and perform activation linking to obtain Compounds as shown.
[0043]
[0044] 2. Catalytic cyclization
[0045] The compound 2 obtained in the above steps was washed twice with DCM (20ml) and DCE (20ml) respectively, then soaked with DCE (8ml) for 30min, and then Grubbs 1 st (25mgx2, 20mg / 0.1mmol) was added to the reaction mixture and shaken at room temperature for 3 hours. The cyc...
Embodiment 2
[0051] The in vitro stability test of embodiment 2 secretin analogs
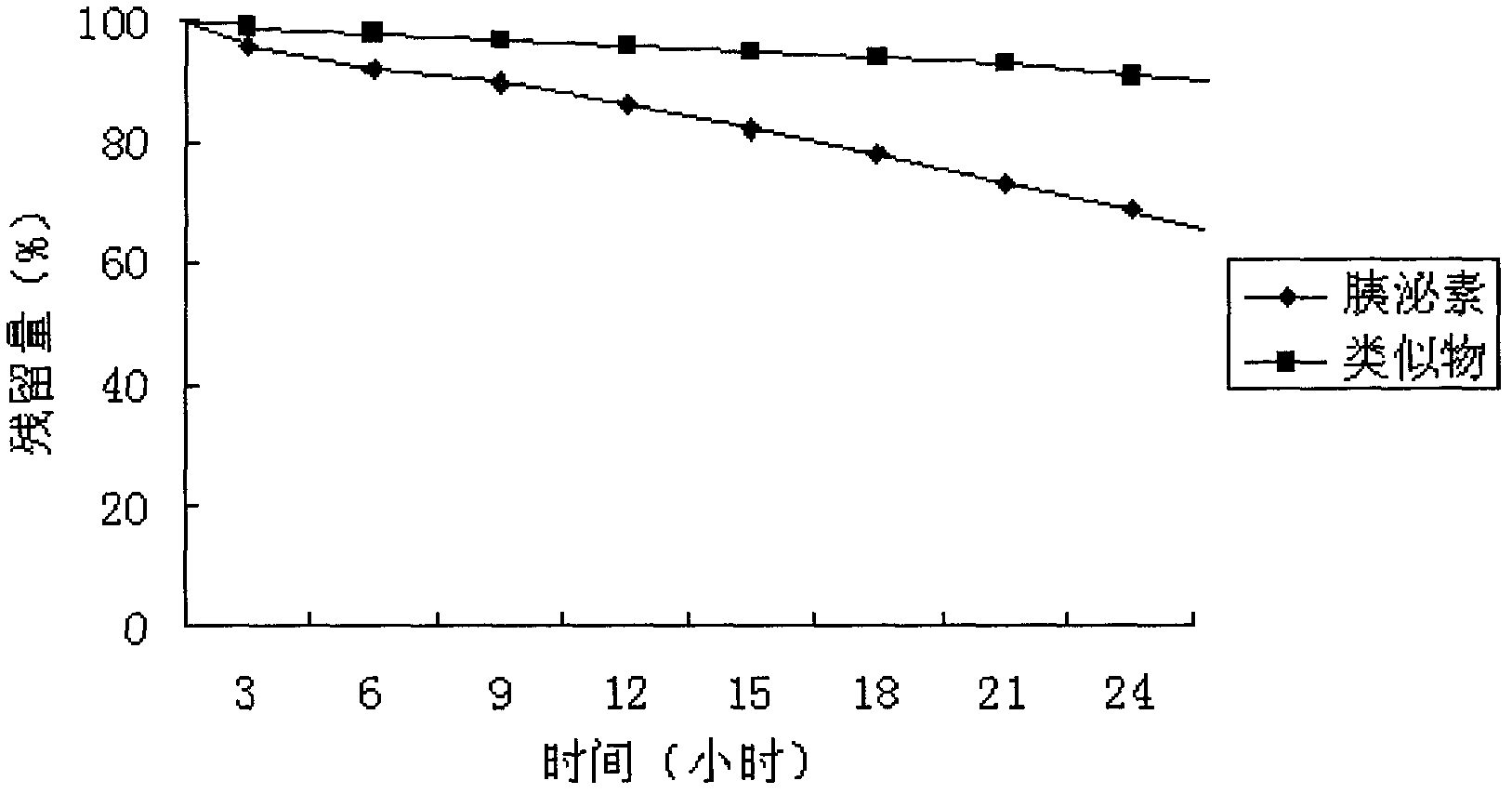
[0052] The natural secretin secretin analogs of the present invention (prepared in Example 1) were placed in 0.1M phosphate buffer (pH7.0), placed at 60°C for 36 hours, and detected by RP-HPLC every three hours Wherein the residual amount of secretin or similar.
[0053] Take samples of secretin and analogues with CH 3 CN and 1% HClO 4 (2:3, v / v) dilution, C 18 The column is the stationary phase, acetonitrile / 0.005M phosphate buffer / 0.2MNaClO 4 It is the mobile phase and detected at 210nm.
[0054] The result is as figure 2 As shown, under the experimental conditions, natural secretin is rapidly degraded, about 30% degraded in 24 hours; while the analogue of the present invention hardly degrades.
Embodiment 3
[0055] The in vitro biological experiment of embodiment 3 secretin analogs
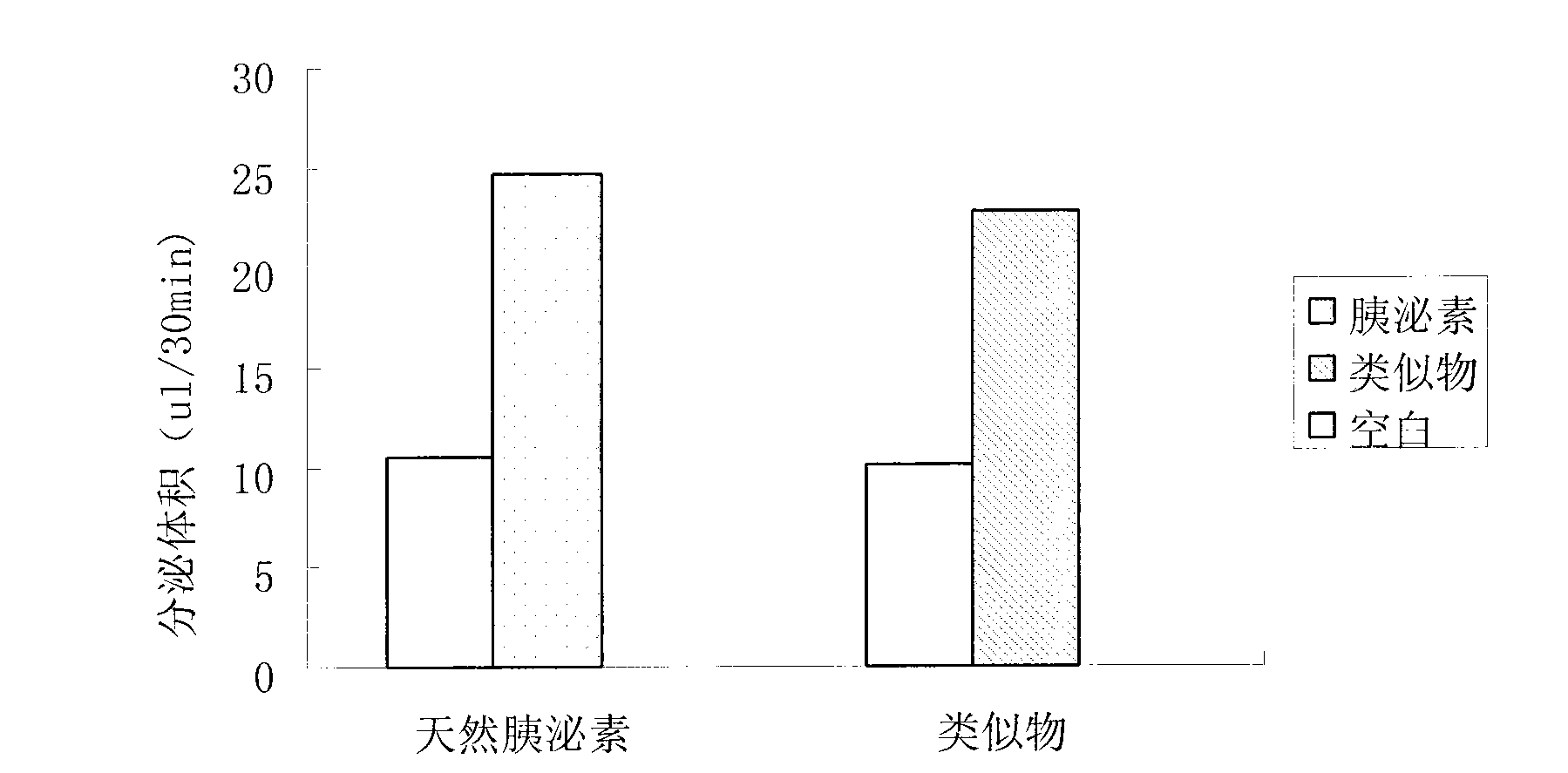
[0056] Ten Wistar rats with a body weight of 200±20 g were selected and randomly divided into two groups (natural secretin group and analog group). Rats were anesthetized with urethane (45 mg / kg), the abdominal cavity was cut open, the pancreas was peeled off, and the isolated pancreas was quickly placed in a constant temperature incubator containing Krebs-Ringer buffer (KR solution) at 37°C. Fill PH7.2 with 95% O with a constant flow pump 2 and 5% CO 2 The KR solution was poured into the abdominal aorta at a constant rate (1.0ml / min), and the buffer solution in the incubation dish was continuously supplied at a rate of 0.35ml / min. The two groups were perfused with 0.25CU / kg.h of natural secretin and the analogue of the present invention (Example 1). A microcapillary was inserted into the pancreatic duct, and the pancreatic juice flowed out along the capillary. The pancreatic juice was collected ev...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com