A kind of degradable polymer composition and preparation method thereof
A technology for degrading polymers and compositions, applied in the field of degradable polymer compositions and their preparation, can solve the problems of poor performance of plant-based polymer materials and difficult degradation of synthetic polymer materials, and achieve controllable service life, The effect of promoting the degradation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Take 100g of polyester (polyethylene adipate) and transition metal salt (copper stearate 2g, silver stearate 0.5g and zinc chloride 0.5g) into a twin-screw mixer, after mixing, The filaments in the molten state are extruded from a spinneret, the filaments are solidified, cooled by water, and then cut into pellet form. Wherein, the rotational speed of the twin-screw mixer is 100-280 rpm.
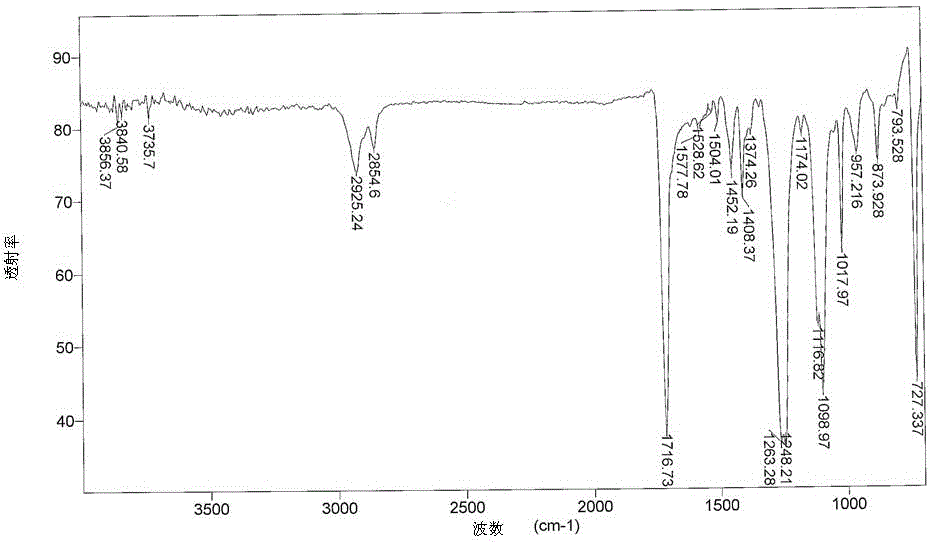
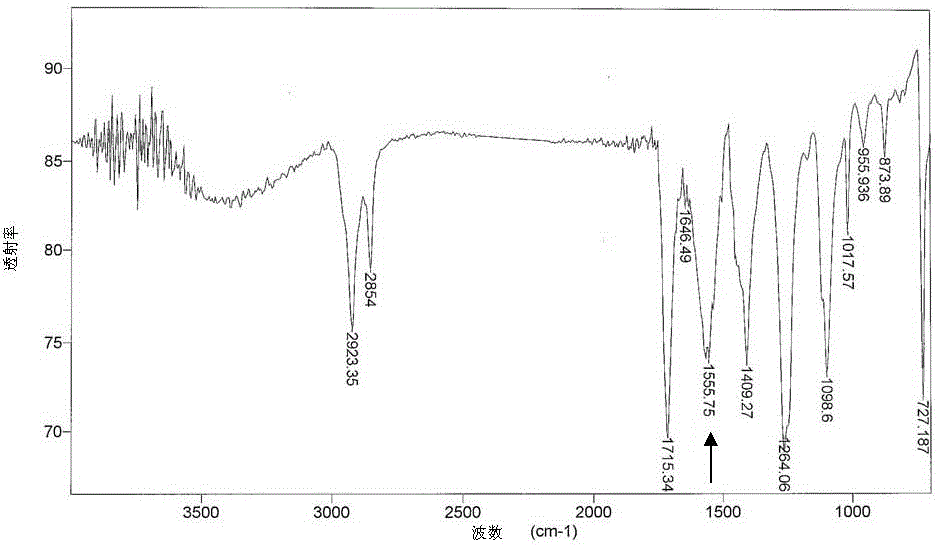
[0078] It was found by FTIR spectroscopy that the carbonyl group (C=O) in the sample structure of the degradable polymer composition prepared in this example was at 1560 cm after 3 days and 10 days of UV treatment -1 The intensity of the absorption band is significantly enhanced (see figure 1 and figure 2 ). The highest intensity of this peak was observed after 14 days of UV irradiation on the degradable polymer composition. According to the literature, the presence of this peak in FTIR reflects the occurrence of chain scission due to photooxidative degradation in the sample.
Embodiment 2
[0080] 100g of the polyester (polyethylene terephthalate), transition metal salts (5g of manganese oleate and 3g of cobalt oleate) and 0.05g of antioxidant IRGANOX® 1010 were added to a twin-screw mixer, and after mixing , the filaments in the molten state are extruded from the spinneret, the filaments are solidified, cooled by water, and then cut into pellets. Wherein, the rotational speed of the twin-screw mixer is 100-280 rpm.
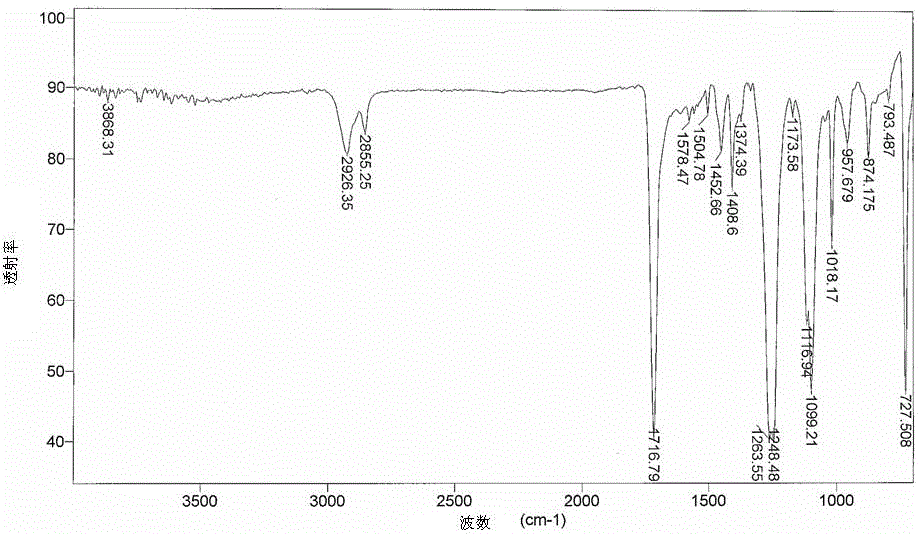
[0081] It was found by FTIR spectroscopy that the carbonyl group (C=O) in the sample structure of the degradable polymer composition prepared in this example was at 1560 cm after 3 days and 10 days of UV treatment -1 The intensity of the absorption band is significantly enhanced (see image 3 and Figure 4 ). The highest intensity of this peak was observed after 14 days of UV irradiation on the degradable polymer composition. According to the literature, the presence of this peak in FTIR reflects the occurrence of chain scission due to photooxid...
Embodiment 3
[0083] 100g of the polyamide (polyphthalamide) and transition metal salt (2.5g copper oleate, 0.05g zinc oleate, 0.5g cerium stearate) were added into a twin-screw mixer, after mixing, the molten state The filaments are extruded from a spinneret, the filaments are solidified, cooled by water, and then cut into pellet form. Wherein, the rotational speed of the twin-screw mixer is 100-280 rpm.
[0084] It was found by FTIR spectroscopy that the carbonyl group (C=O) in the sample structure of the degradable polymer composition prepared in this example was at 1736 cm after 3 days and 10 days of UV treatment. -1 The intensity of the absorption band is significantly enhanced (see Figure 5 and Figure 6 ). The highest intensity of this peak was observed after 14 days of UV irradiation on the degradable polymer composition. According to the literature, the presence of this peak in FTIR reflects the occurrence of chain scission due to photooxidative degradation in the sample.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com