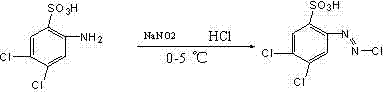Industrial production method of environment-friendly yellow color lake pigment 183 for plastics
A production method and an environmentally friendly technology, applied in the direction of organic dyes, etc., can solve the problems of poor color development, difficult unloading, hard pigment blocks, etc., and achieve low hydrophilicity, convenient unloading and high tinting strength. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
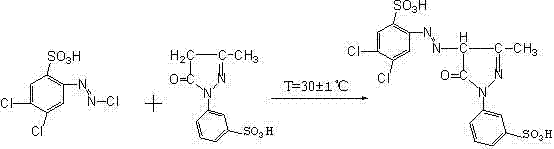
[0029] 1) Add 246Kg of 2-amino-4,5-dichlorobenzenesulfonic acid to the reaction kettle, add 2000Kg of water and 140Kg of liquid caustic soda with a concentration of 30wt% (the concentration of the liquid caustic soda in the following examples is the same as this example), and heat up to 30-35 ℃, stirring and reacting for 1 hour; slowly adding concentrated hydrochloric acid at 15±1℃, stirring and reacting for 30min; Sulfamic acid is removed to obtain diazonium liquid;
[0030] 2) Add 2000Kg of water, 256Kg of liquid caustic soda and 150Kg of sodium acetate into the reaction kettle, stir to dissolve, then add 271Kg of 1-(3'-sulfonic acid phenyl)-3-methyl-5-pyrazolone at 30 Stir at a temperature of ~35°C to obtain a clear liquid;
[0031] 3) Inject the diazo solution into the coupling reaction kettle at 30±1°C for coupling reaction, and stir for 60 minutes after adding the diazo solution; raise the temperature of the reaction solution to 60±1°C, and add 30% of the reaction solut...
Embodiment 2
[0034] 1) Add 246Kg of 2-amino-4,5-dichlorobenzenesulfonic acid into the reaction kettle, add 2000Kg of water and 140Kg of liquid caustic soda, heat up to 30-35°C, stir and react for 1 hour; slowly add concentrated hydrochloric acid at 15±1°C, Stir the reaction for more than 30 minutes; then slowly add 400Kg of 30% sodium nitrite aqueous solution at 0°C to carry out the diazotization reaction and stir for 1 hour, and remove the excess sodium nitrite with sulfamic acid to obtain the diazonium solution;
[0035] 2) Add 2000Kg of water, 256Kg of liquid caustic soda and 150Kg of sodium acetate into the reaction kettle, stir to clear, then add 295Kg of 1-(3'-sulfonic acid phenyl)-3-methyl-5-pyrazolone, at 30 Stir at a temperature of ~35°C to obtain a clear liquid;
[0036] 3) Inject the diazo solution into the coupling reaction kettle at 30±1°C for coupling reaction, and stir for 60 minutes after adding the diazo solution; raise the temperature of the reaction solution to 60±1°C, a...
Embodiment 3
[0038]1) Add 246Kg of 2-amino-4,5-dichlorobenzenesulfonic acid into the reaction kettle, add 2000Kg of water and 140Kg of liquid caustic soda, heat up to 30-35°C, stir and react for 1 hour; slowly add concentrated hydrochloric acid at 15±1°C, Stir the reaction for more than 30 minutes; then slowly add 400Kg of 30% sodium nitrite aqueous solution at 0°C to carry out the diazotization reaction and stir for 1 hour, and remove the excess sodium nitrite with sulfamic acid to obtain the diazonium solution;
[0039] 2) Add 2000Kg of water, 256Kg of liquid caustic soda and 150Kg of sodium acetate into the reaction kettle, stir to clear, then add 396Kg of 1-(3'-sulfonic acid phenyl)-3-methyl-5-pyrazolone, at 30 Stir at a temperature of ~35°C to obtain a clear liquid;
[0040] 3) Inject the diazo solution into the coupling reaction kettle at 30±1°C for coupling reaction, and stir for 60 minutes after adding the diazo solution; raise the temperature of the reaction solution to 60±1°C, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com