Potassium aspartate pharmaceutical composition and preparation method thereof
A technology of potassium aspartate and magnesium aspartate, which is applied in the field of pharmaceutical compositions, and can solve the problem of inability to complete capsule and powder filling and tablet compression process, poor fluidity of potassium aspartate agglomerated particles , environmental humidity and high requirements for pharmaceutical equipment, etc., to achieve the effect of stable and reliable product quality, reasonable proportion and good compressibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、2、3、5、6、9
[0097] Embodiment 1,2,3,5,6,9 preparation method is:
[0098] a. Take potassium aspartate or magnesium aspartate and micropowder silica gel, pulverize
[0099] b. Pass through a 100-mesh sieve, add microcrystalline cellulose, starch, croscarmellose sodium, magnesium stearate, and mix well
[0100] c. Configure 5% ethyl cellulose ethanol solution as a binder
[0101] d. Add an appropriate amount of binder to make soft materials, pass through a 20-mesh sieve and granulate
[0102] e. Dry the wet granules in a blast oven at 60°C for 1 hour, and sort through a 18-mesh sieve
[0103] f. Inspection of semi-finished products, with Die pressing
[0104] g. Film-coat the plain tablets, the coating powder is Colorcon OY-S-28827, to increase the weight by 2-3%
[0105] h. Aluminum-plastic packaging, ready to use
Embodiment 4
[0106] Embodiment 4 preparation method is:
[0107] a. Take potassium aspartate or micronized silica gel, pulverize
[0108] b. Pass through a 100-mesh sieve, add microcrystalline cellulose, and mix well
[0109] c. configure 5% povidone ethanol solution as adhesive
[0110] d. Add an appropriate amount of binder to make soft materials, pass through a 20-mesh sieve and granulate
[0111] e. Dry the wet granules in a blast oven at 60°C for 1 hour, sort through a 18-mesh sieve, add croscarmellose sodium and magnesium stearate, and mix
[0112] f. Inspection of semi-finished products, with Die pressing
[0113] g. Film-coat the plain tablets, the coating powder is Colorcon OY-S-28827, to increase the weight by 2-3%
[0114] h. Aluminum-plastic packaging, ready to use
Embodiment 7、 Embodiment 8
[0115] Embodiment 7, embodiment 8 preparation method are:
[0116] a. Mix potassium aspartate with micropowder silica gel and pulverize
[0117] b. Through 100 mesh sieve
[0118] c. Configure 5% ethyl cellulose ethanol solution as a binder
[0119] d. Add an appropriate amount of binder to make a suitable soft material, pass through a 20-mesh standard sieve, and granulate
[0120] e. Wet granules are dried at 60°C±5°C and crushed through a 60-mesh sieve
[0121] f. Inspection of semi-finished products
[0122] g. Aluminum foil bags are directly subpackaged (Example 7, granules) or filled with capsules (Example 8, Capsules), and packed in aluminum and plastic, to get final product.
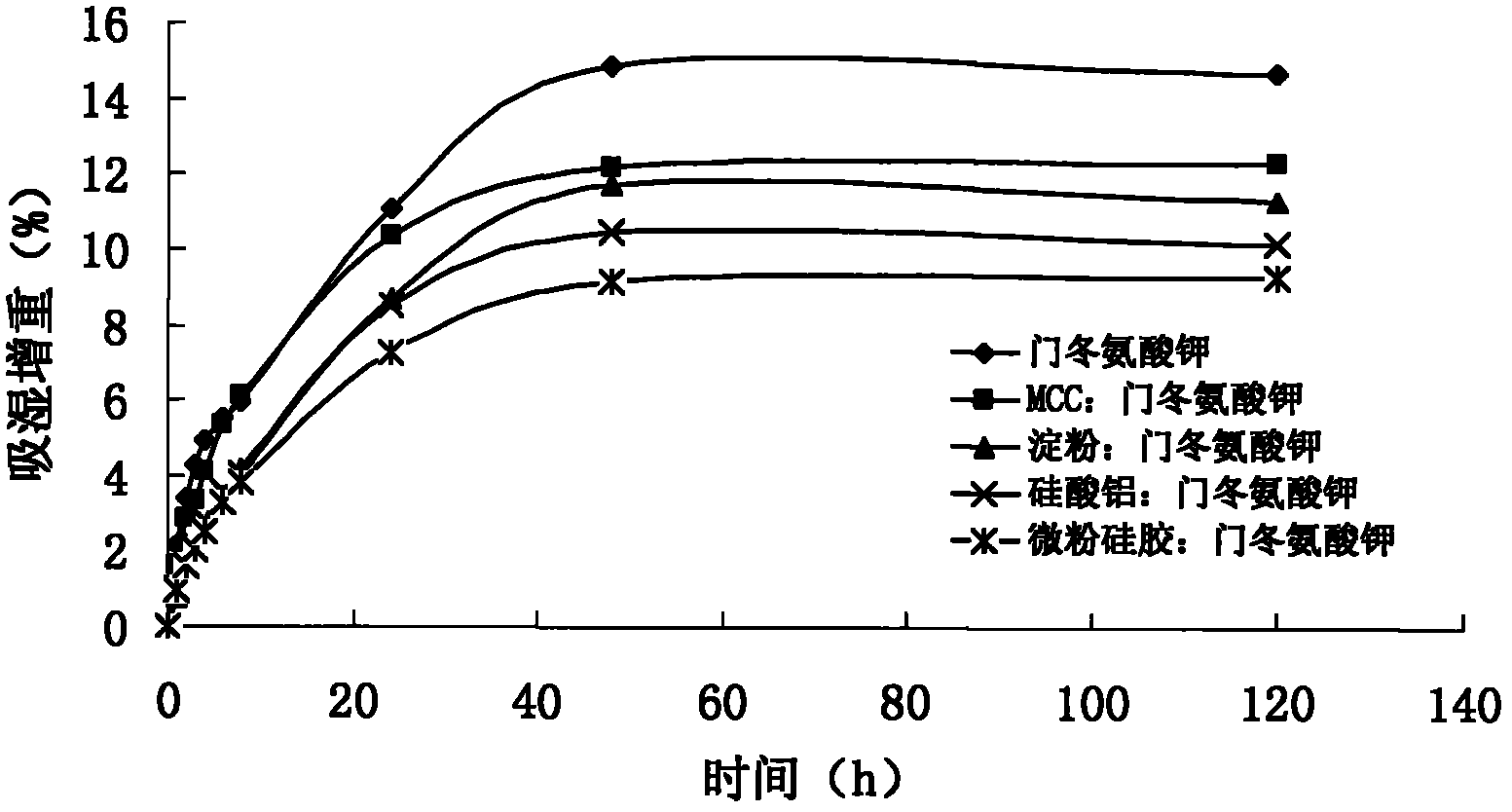
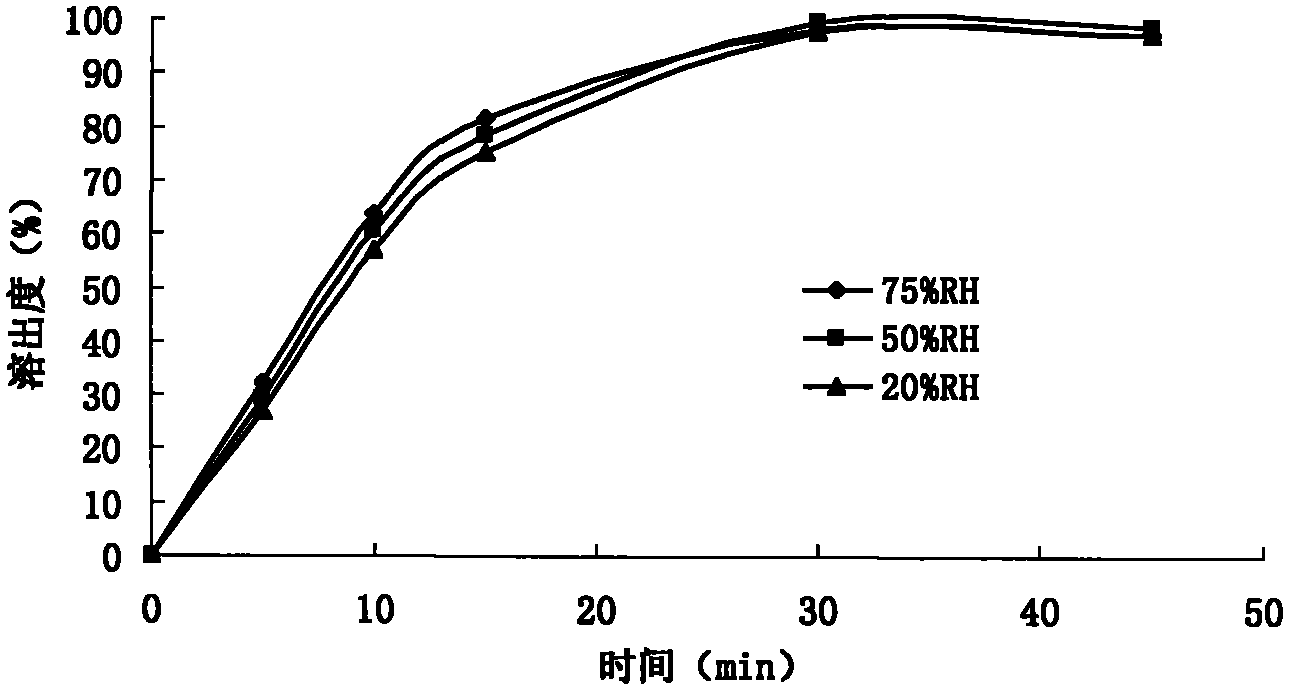
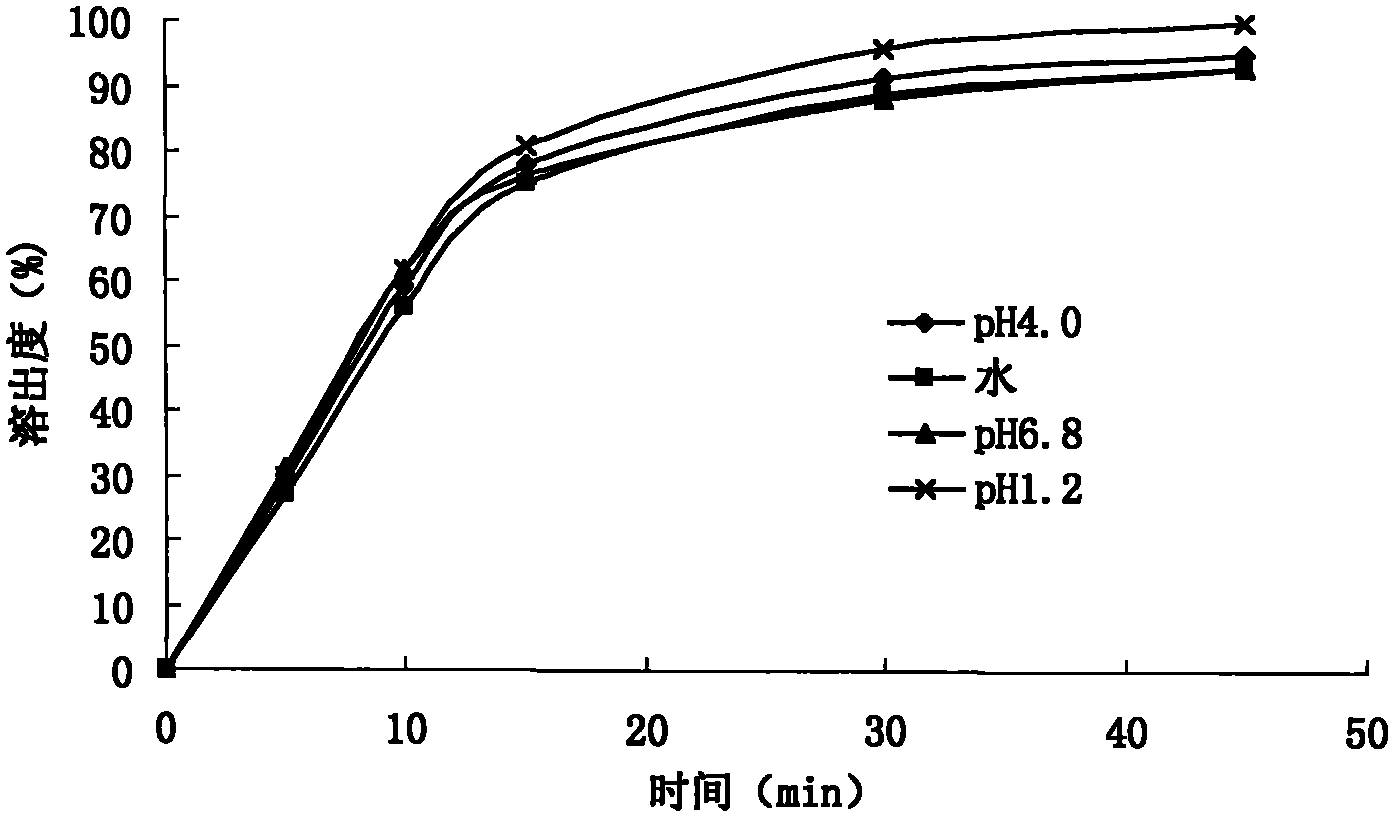
[0123] The embodiment sample prepared under humidity 60%RH environment, according to Chinese Pharmacopoeia 2010 edition appendix XIX C stability test guiding principle, carries out influence factor experiment: sample removes aluminum-plastic packaging, respectively under strong light irradiati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com