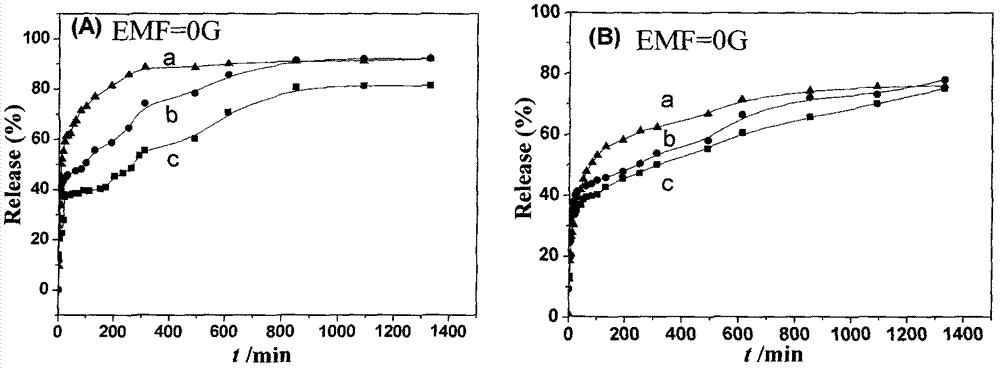
Tegafur/layered double hydroxide (TF/LDHs) nanohybrid-magnetic matrix compound and preparation method thereof
A nano-hybrid, magnetic matrix technology, applied in the direction of drug combination, medical preparations containing active ingredients, drug delivery, etc., can solve the problems of leukocyte and thrombocytopenia, and achieve the effect of mild reaction and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] a. 5.95g (0.02mol) Zn (NO 3 ) 2 ·6H 2 O and 3.75g (0.01mol) Al(NO 3 ) 3 9H 2 O was dissolved in 150 mL deionized water.
[0032] b. The magnetic matrix MgFe 2 o 4 Dissolved in the mixed salt solution prepared in step a, the mass ratio of the magnetic matrix to the trivalent metal salt is 1:50;
[0033] c. preparation concentration is the NaOH solution of 0.3mol / L;
[0034] d. Dissolve 1.23 g of tegafur in the aqueous alkali solution prepared in step c to prepare a tegafur solution with a concentration of 0.08 mol / L.
[0035] e. Add the solution of step d to the solution of step b, stir and control the pH to 10, the reaction temperature is 20°C; the reaction time is 3 hours, and then the resulting slurry is aged at 20°C for 36 hours, filtered, and washed with water until neutral , dried at 60°C to obtain MgFe 2 o 4 (TF / LDHs) nanocomposites.
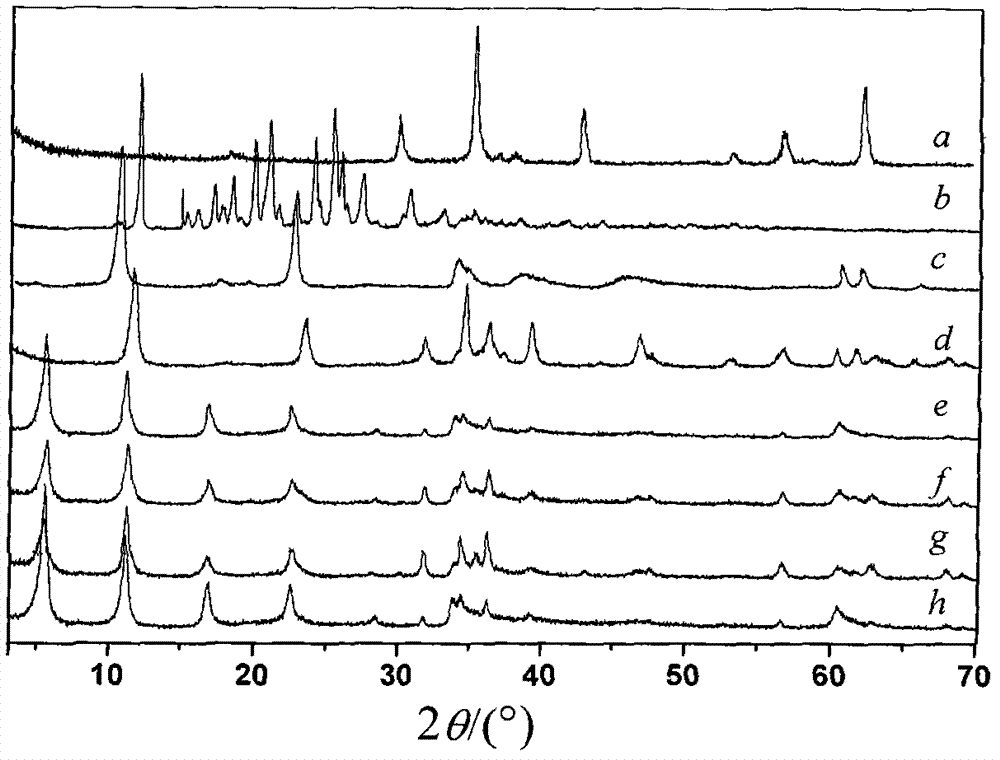
[0036] By XRD spectrum ( figure 1 f) It can be seen that the MgFe 2 o 4 (TF / LDHs) nanocomposites have a layered cry...
Embodiment 2
[0038] a. 5.95g (0.02mol) Zn (NO 3 ) 2 ·6H 2 O and 3.75g (0.01mol) Al(NO 3 ) 3 9H 2 O was dissolved in 150 mL deionized water.
[0039] b. The magnetic matrix MgFe 2 o 4 Dissolved in the mixed salt solution prepared in step a, the mass ratio of the magnetic matrix to the trivalent metal salt is 1:20;
[0040] c. Prepare NaCO with a concentration of 3mol / L 3 solution;
[0041] d. Dissolve 0.50 g of tegafur in the aqueous alkali solution prepared in step c to prepare a tegafur solution with a concentration of 0.02 mol / L.
[0042] e. Add the solution of step d to the solution of step b, stir and control the pH to 12, the reaction temperature is 80°C; the reaction time is 1 hour, and then the resulting slurry is aged at 80°C for 10 hours, filtered, and washed with water until neutral , dried at 60°C to obtain MgFe 2 o 4 (TF / LDHs) nanocomposites.
[0043] By XRD spectrum ( figure 1 g) It can be seen that the MgFe 2 o 4 (TF / LDHs) nanocomposites have a layered crystal...
Embodiment 3
[0046] a. 5.95g (0.02mol) Zn (NO 3 ) 2 ·6H 2 O and 3.75g (0.01mol) Al(NO 3 ) 3 9H 2 O was dissolved in 150 mL deionized water.
[0047] b. The magnetic matrix MgFe 2 o 4 Dissolved in the mixed salt solution prepared in step a, the mass ratio of the magnetic matrix to the trivalent metal salt is 1:10;
[0048] c. preparation concentration is the NaOH solution of 0.5mol / L;
[0049] d. Dissolve 1.40 g of tegafur in the aqueous alkali solution prepared in step c to prepare a tegafur solution with a concentration of 0.09 mol / L.
[0050] e. Add the solution of step d to the solution of step b, stir and control the pH to 11, and the reaction temperature is 65°C; the reaction time is 2 hours, then the resulting slurry is aged at 65°C for 24 hours, filtered, and washed with water until neutral , dried at 60°C to obtain MgFe 2 o 4 (TF / LDHs) nanocomposites.
[0051] By XRD spectrum ( figure 1 h) It can be seen that the MgFe 2 o 4 (TF / LDHs) nanocomposites have a layered cry...
PUM
| Property | Measurement | Unit |
|---|---|---|
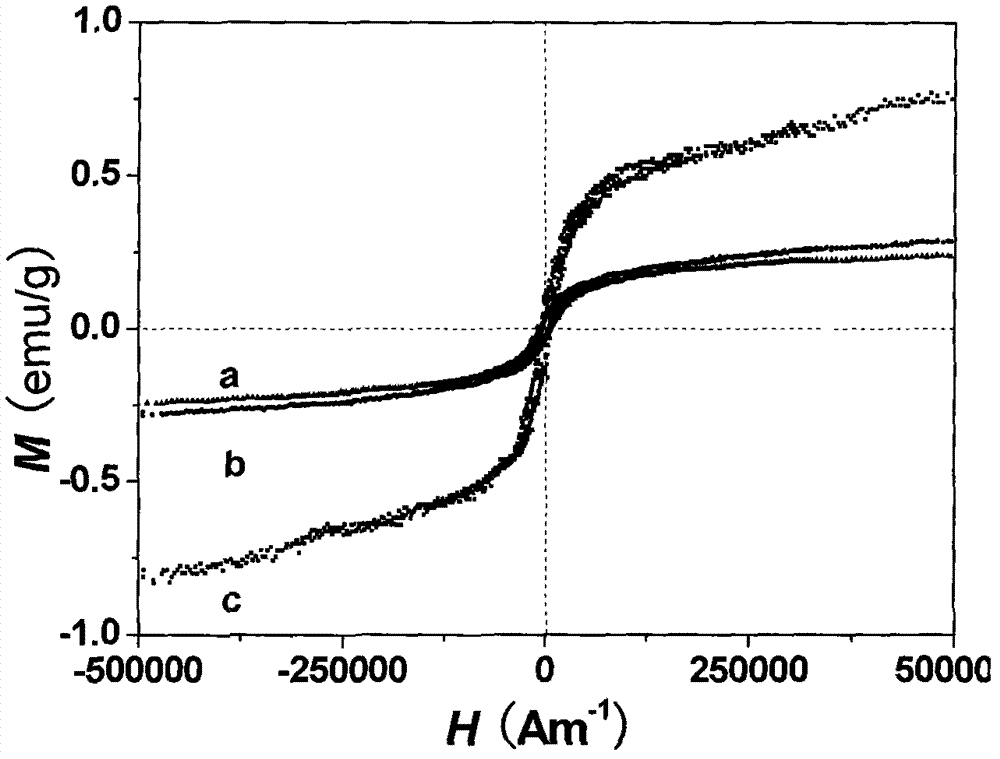
| Specific saturation magnetization | aaaaa | aaaaa |
| Coercivity | aaaaa | aaaaa |
| Specific saturation magnetization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com