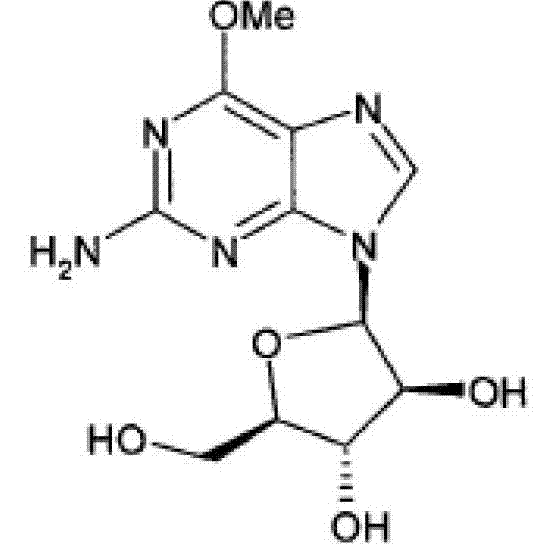
Nelarabine injection composition and preparation method thereof
The technology of a composition and injection, which is applied in the field of medicine, can solve the problems of crystallization, blockage of microcirculation, and damage to health of nelarabine injection, and achieve the effect of simple and feasible preparation method, reduction of precipitation, and solution of crystallization problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] [Example 1] Preparation of Nelarabine Crystalline Compound
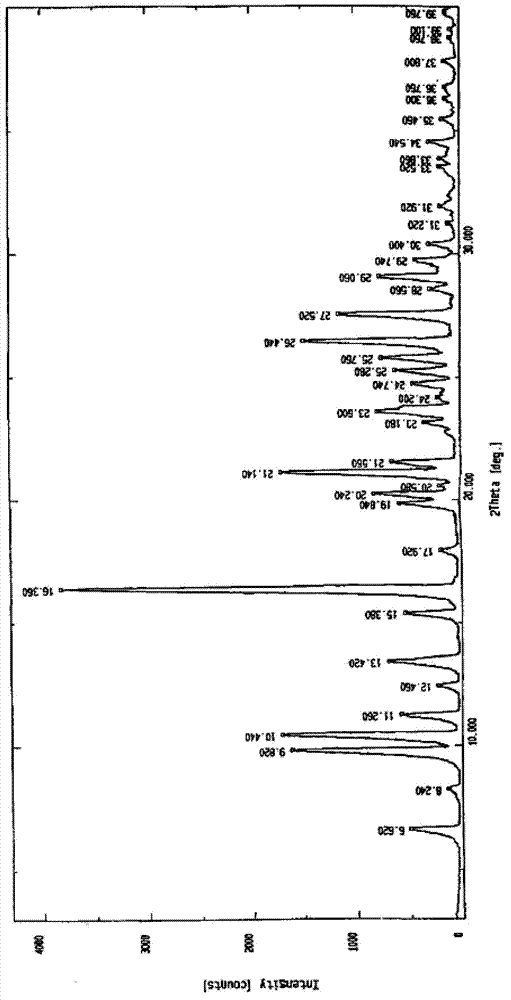
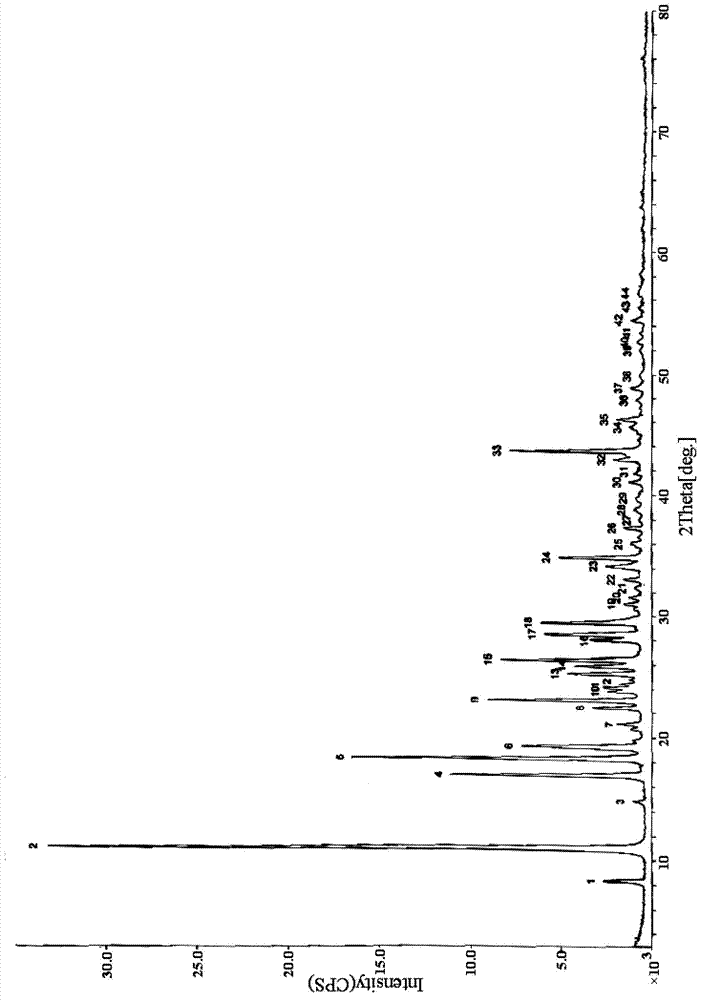
[0043] Get 25.0 g of the nalarabine crude product and put it into 800 ml of anhydrous methanol, heat to reflux for 2 hours, the nalarabine crude product is basically dissolved, then add 1 gram of activated carbon and reflux for 15 minutes, filter while it is hot, drop to room temperature, and cool to -5°C, crystallize, filter with suction, wash the filter cake with a small amount of ice methanol to obtain 11.6 g of fine nelarabine, which is the crystalline compound of nelarabine.
[0044] The obtained nelarabine crystalline compound is subjected to the following X-ray powder diffraction analysis:
[0045] Sample treatment: the prepared nelarabine crystalline compound was ground and passed through a 100-mesh sieve, and 50 mg was weighed as a sample for powder X-ray diffraction experiment.
[0046] Experimental instrument: Japan Rigaku D / max-2550 powder X-ray diffractometer.
[0047] Experimental conditions: C...
Embodiment 2
[0053] [embodiment 2] preparation of nelarabine crystalline compound
[0054] Take 25.0 g of crude nalarabine and put it into 750 ml of anhydrous methanol, heat to reflux, stir for 3 hours, and the nalarabine is basically dissolved, then add 1 g of activated carbon and reflux for 10 minutes, filter while it is hot, drop to room temperature, and cool down in an ice-salt bath At 0°C, crystallize, filter with suction, and wash the filter cake with a small amount of ice methanol to obtain 11.8 g of fine nelarabine, which is the crystalline compound of nelarabine.
Embodiment 3
[0055] [Example 3] Preparation of Nelarabine Crystalline Compound
[0056] Take 25.0 g of nalarabine crude product and put it into 875 ml of anhydrous methanol, heat to reflux, stir for 1 hour, and the nalarabine is basically dissolved, then add 1 g of activated carbon and reflux for 20 minutes, filter while it is hot, drop to room temperature, and cool down in an ice-salt bath At -3°C, crystallize, filter with suction, and wash the filter cake with a small amount of ice methanol to obtain 11.9 g of fine nelarabine, which is the crystalline compound of nelarabine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com