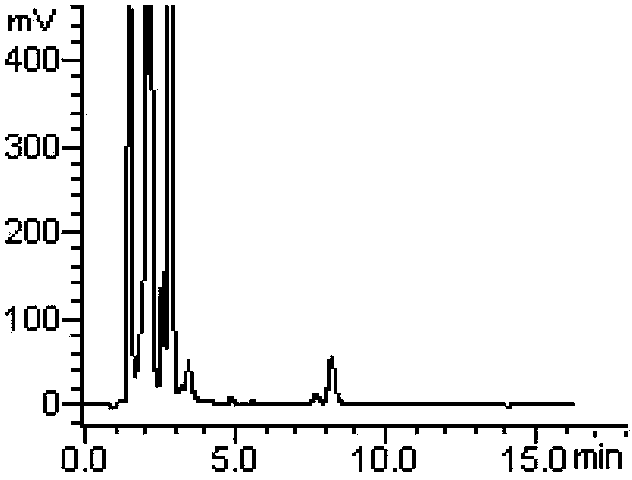
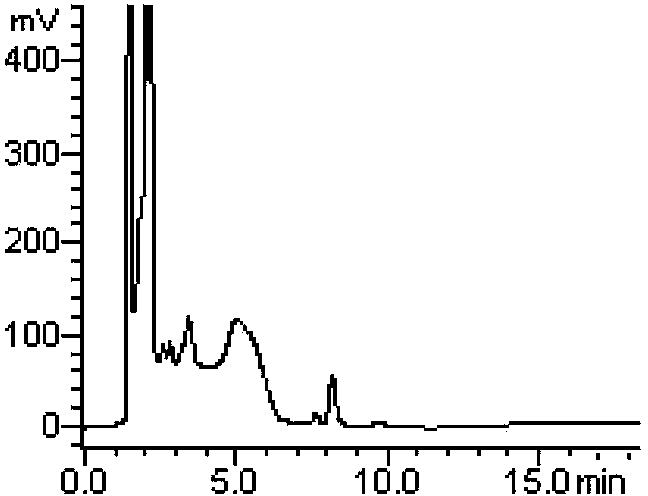
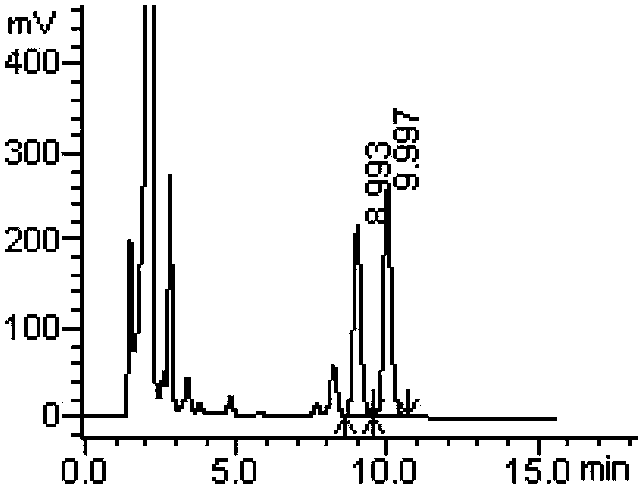
Method for measuring derivatization in valproic acid body fluid concentration process through high performance liquid chromatography
A technology of high performance liquid chromatography and valproic acid, which is applied in the field of derivatization in the process of determining the concentration of valproic acid in body fluids by high performance liquid chromatography, and can solve problems such as shortening the life of the chromatographic column, poor repeatability, and incomplete reaction , to achieve the effect of no endogenous impurity interference, accurate measurement results and prolonging the service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: A derivatization method in the process of measuring the body fluid concentration of valproic acid by high performance liquid chromatography, using tetramethylammonium hydroxide as a catalyst and 2,4'-dibromoacetophenone as a derivatizing agent.
[0035] The reaction formula is as follows:
[0036]
[0037] Specific operation steps: Accurately take 50 μL of quality control or patient serum, add 60 μL of 0.10 μg / μL internal standard pelargonic acid solution, add 50 μL of 3mol / L sulfuric acid solution, and mix well; add 2 mL of diethyl ether, vortex extract for 1 min, and centrifuge for 5 min (3500r· min -1 ), draw the supernatant into a centrifuge tube, add 100 μL of 10 mmol / L tetramethylammonium hydroxide acetonitrile solution and vortex to mix, then add 63 μL of 2 mg / mL 2,4′-dibromoacetophenone acetonitrile solution (that is, the derivatizer is 126μg, 0.45μmol, which is equivalent to 5 times the molar multiple of the reactant, and the concentration of valpr...
Embodiment 2
[0046] Example 2: A derivatization method in the process of measuring the body fluid concentration of valproic acid by high performance liquid chromatography, using tetramethylammonium hydroxide as a catalyst and α-bromoacetophenone as a derivatizing agent.
[0047] The reaction formula is as follows:
[0048]
[0049] Specific operation steps: Accurately take 50 μL of quality control or patient serum, add 50 μL of 0.16 μg / μL internal standard cyclohexyl propionic acid solution, add 50 μL of 3mol / L sulfuric acid, and mix well; add 2 mL of ether, vortex extract for 1 min, centrifuge for 5 min (3500r / min), draw the supernatant into a centrifuge tube, add 100 μL of 10 mmol / L tetramethylammonium hydroxide acetonitrile solution, vortex and mix well, then add 55 μL of 2 mg / mL α-bromoacetophenone acetonitrile solution (that is, the derivatizer is 110 μg, 0.55μmol, which is equivalent to 5.3 times the molar multiple of the reactant, and the concentration of valproic acid in the re...
Embodiment 3
[0058] Example 3: A derivatization method in the process of measuring the body fluid concentration of valproic acid by high performance liquid chromatography, using tetrabutylammonium hydroxide as a catalyst and α-bromoacetophenone as a derivatizing agent.
[0059] The reaction formula is as follows:
[0060]
[0061] Specific operation steps: Accurately take 50 μL of quality control or patient serum, add 50 μL of 0.16 μg / μL internal standard cyclohexyl propionic acid solution, add 50 μL of 3mol / L sulfuric acid, and mix well; add 2 mL of ether, vortex extract for 1 min, centrifuge for 5 min (3500r / min), draw the supernatant into a centrifuge tube, add 40 μL of 10 mmol / L tetrabutylammonium hydroxide acetonitrile solution, vortex mix for 30 s, then add 55 μL of 2 mg / mL α-bromoacetophenone acetonitrile solution (that is, the derivatizer is 110 μg, 0.55 μmol, equivalent to 5.3 times the molar multiple of the reactant, the concentration of valproic acid in the reactant serum is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com