Flavone derivatives and their preparative method and medical use
一种衍生物、黄酮的技术,应用在新黄酮衍生化合物领域,能够解决临床意义不大、作用剂量大、缺乏阳性对照药等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
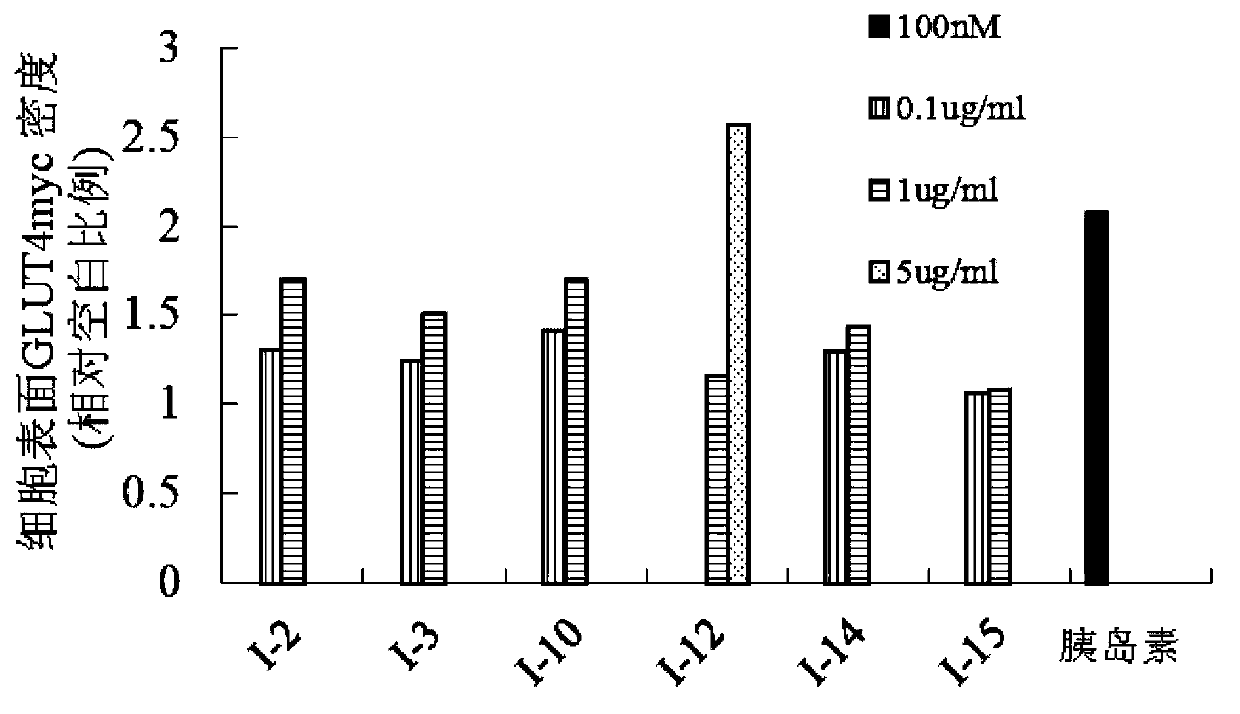
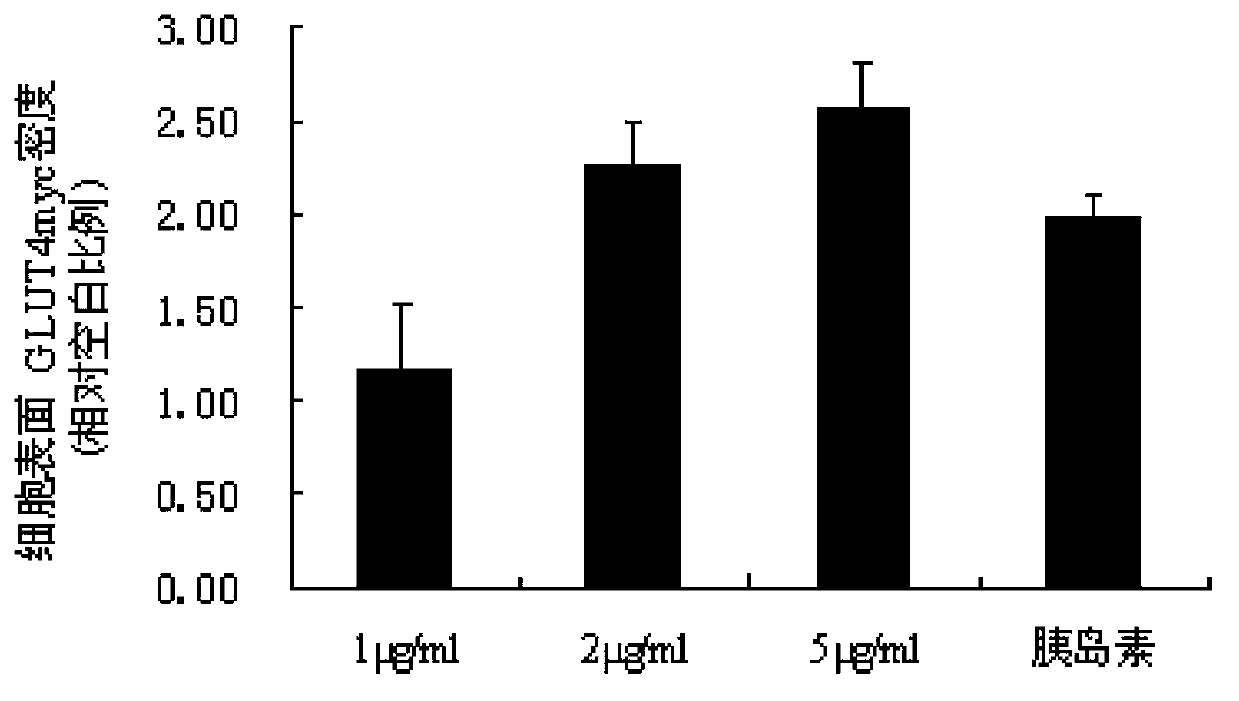
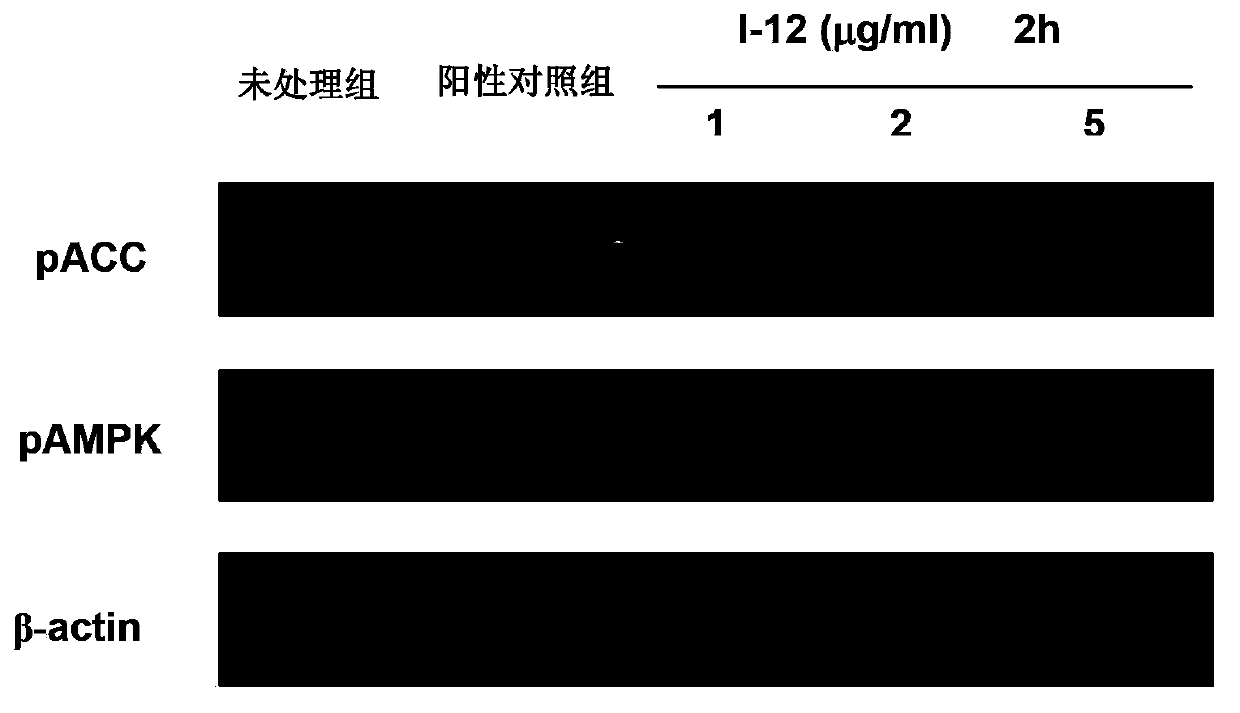
Image
Examples
Embodiment 1
[0037] Synthetic General Method of Series Compound II
[0038]Weigh an appropriate amount of substituted benzaldehyde into a reaction flask, add excess acetone, and stir at room temperature to fully dissolve the raw materials. Add 1.5 equivalents of 4 mol / L sodium hydroxide solution to the reaction flask at room temperature, and stir the reaction solution at 40°C. TLC monitored until the reaction was complete. The reaction solution was concentrated under reduced pressure to remove excess acetone. Add 1 mol / L dilute hydrochloric acid to the residue to adjust the pH to be slightly acidic. Extract with appropriate amount of dichloromethane 3 times, and combine the organic phases. The organic phase was dried with anhydrous magnesium sulfate, filtered, and concentrated to obtain a crude product, which was subjected to flash silica gel column chromatography to obtain a series of compounds II shown in Formula 4.
[0039]
[0040] Formula 4
Embodiment 2
[0042] Synthetic general method of series compound III:
[0043] Weigh an appropriate amount of compound II into a reaction flask, and add tetrahydrofuran to dissolve it. 1.2 equivalents of pyrrolidone hydrotribromide (PHT) was weighed and dissolved in an appropriate amount of tetrahydrofuran, and the solution was slowly dropped into the reaction flask at room temperature. After the dropping, it was stirred at room temperature for 24 h, or monitored by TLC until the reaction was complete. After the reaction was completed, the white insoluble matter was removed by filtration, and the filtrate was collected. The tetrahydrofuran in the filtrate was removed under reduced pressure, and the concentrate was subjected to flash silica gel column chromatography to obtain a series of compounds III shown in Formula 5.
[0044]
[0045]
[0046] Formula 5
Embodiment 3
[0048] Synthetic general method of series compound I:
[0049] Weigh kaempferol (1.2 equivalents), anhydrous K 2 CO 3 (1.2 equivalents) in the three-necked bottle, add dioxane 5ml to fully dissolve kaempferol, after the reaction solution was stirred for 1.5h under reflux, compound III (1 equivalent) was dissolved in 2ml dioxanes, and the The solution was added dropwise to the reaction solution under reflux, and the drop was completed in about half an hour. The reaction solution continued to reflux. After the disappearance of compound III was monitored by TLC, the reaction solution was cooled to room temperature, dioxane was removed under reduced pressure, dilute hydrochloric acid was added to the residue to adjust the pH value to acidity, extracted with ethyl acetate, and the organic phase was dried with anhydrous magnesium sulfate , filtered and concentrated. The crude product was separated by HW-40c gel column chromatography, dichloromethane-methanol (1:1, V / V) was used ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com